- Home Page
- Company Profile
-
Our Products
- Active Pharmaceutical Ingredient
- (R)-Salbutamol Hydrochloride
- Pharmaceutical pellets
- Veterinary APIs
- Pharmaceuticals impurities
- 10 Hydroxycamptothecin
- 11-Alpha-Acetoxyprogesterone
- Aceglutamide
- 6-Dehydronandrolone Acetate
- Abacavir
- 3 Hydroxy 4 Amino Butyric Acid
- Acebrophylline
- 2,4-Dichlorobenzoic Acid
- 2',3'-Dideoxyuridine
- 2-Methoxyestradiol
- 3-Hydroxy-4-amino-butyric Acid
- 2-Nitroaminoimidazoline
- 3-O-Ethyl-L-ascorbic Acid
- 5-Androstenediol
- 5,7-Dichloro-8-hydroxyquinoline
- 5-Fluorouracil
- 5-hydroxy Tryptophan
- 5-Methyl-2'-deoxycytidine
- 8(4-Sulfophenyl)Theophylline
- 15S-Cloprostenol
- 1000px-Aspirin-skeletal.svg
- Abacavir Hydrochloride
- Abamectin
- Abarelix
- Abiraterone
- Abiraterone Acetate
- Acamprosate Calcium
- Acarbose
- Acebutolol
- Aceclofenac
- Acefylline Piperazine
- Acenocoumarol
- Acepifylline
- Acesulfame K
- Acetamide
- Acetazolamide
- Acexamic Acid
- Aciclovir Sodium
- Acipimox
- Acofide trihydrate
- Acotiamide
- Acriflavine hydrochloride
- Actinospectacin sulfate
- Adamantane
- Adapalene
- Adefovir
- Adefovir dipivoxil
- Ademetionine
- Ademetionine 1,4-butanedisulfonate
- Ademetionine disulfate tosylate
- Adenine
- Adenosine
- Adenosine triphosphate
- Adrenaline
- Afatinib Base
- Afatinib dimaleate
- Agomelatine
- Aica hydrochloride
- Alacepril
- Alanyl-glutamine
- Alarelin
- Albendazole
- Alendronate sodium trihydrate
- Alfuzosin
- Alfuzosin hydrochloride
- Algestone acetophenide
- Aliskiren
- Alizapride hydrochloride
- Almotriptan Maleate
- Alogliptin benzoate
- Alovudine
- Alpha-Asarone
- alpha-Ketovaline calcium
- Alpha Picolinic acid
- Altizide
- Altrenogest
- Amiloride hydrochloride
- Amitraz
- Amlexanox
- Amlodipine hydrochloride
- Ammonium bifluoride
- Ammonium fluoride
- Amodiaquine hydrochloride
- Amorolfine hydrochloride
- Amoxicillin Trihydrate
- Amphenidone
- Ampicillin
- Ampicillin sodium
- Ampicillin Trihydrate
- Amrinone
- Amygdalin
- Amyl Meta Cresol
- Anacetrapib
- Anastrozole Ingredient
- Anidulafungin
- Anisomycin
- Ansamitocin
- Antazoline Hydrochloride
- Antazoline Phosphate
- Antide
- Apomorphine Hydrochloride
- Apremilast
- Arformoterol Tartrate
- Argireline
- Aripiprazole
- Artemether.svg
- Artemisinin
- Articaine
- Articaine Hydrochloride
- Ascorbic Acid
- Ascorbyl Palmitate
- Asenapine
- Asenapine Maleate
- Aspartame
- Asunaprevir
- Atorvastatin Calcium
- Atorvastatin Calcium Trihydrate
- Atosiban
- Atosiban Acetate
- Atovaquone
- Atracurium
- Atracurium Besylate
- Atropine Sulfate
- Avatrombopag
- Avermectin
- Avibactam
- Avibactam Sodium
- Aviptadil
- Axitinib
- Azamethiphos
- Azasetron
- Azasetron hydrochloride
- Azathioprine
- Azathramycin
- Azelastine hydrochloride
- Azilsartan kamedoxomil
- Azilsartan medoxomil
- Azithromycin dihydrate
- Azlocillin sodium
- Azosemide
- Aztreonam
- Baccatine III
- Bacitracin Methylene Disalicylate
- Baicalin
- Balofloxacin
- Balsalazide disodium dihydrate
- Bambuterol hydrochloride
- Bamifylline Hydrochloride
- Bazedoxifene
- Bazedoxifene acetate
- Bedaquiline
- Bedaquiline fumarate
- Bendamustine
- Bendamustine hydrochloride
- Bendazol
- Bendroflumethiazide
- Benfotiamine
- Bengenin
- Benidipine hydrochloride
- Benproperine phosphate
- Benserazide hydrochloride
- Benzalkonium chloride
- Benzarone
- Benzbromarone
- Benzethonium Chloride
- Benzidamine hydrochloride
- Benziodarone
- Benzobarbital
- Benzocaine
- Benzonatate
- Benzphetamine Hydrochloride
- Benzthiazide
- Benztropine mesylate
- Benzylpenicilline Benzathine
- Benzyl Alcohol
- Benzylpenicillin Potassium
- Bepotastine Besilate
- Beraprost
- Beraprost Sodium
- Berberine hydrochloride
- Besifloxacin hydrochloride
- beta-Carotene
- Betahistine
- Betahistine dihydrochloride
- Betahistine Hydrochloride
- Betaine Citrate Anhydrous
- Betamethasone 17-valerate
- Betamethasone acetate
- Betamethasone Dipropionate
- Betamethasone Sodium Phosphate
- Betamethasone Valerate
- Betaxolol
- Hydroxycamptothecin
- Pilocarpine Hydrochloride
- Salbutamol hydrochloride
- Betaxolol hydrochloride
- Betulinic Acid
- Bevantolol Hydrochloride
- Bezafibrate
- Bicalutamide
- Bietamiverine
- Bifendatatum
- Bifendate
- Bifonazole
- Bilastine
- Bilirubin
- Biperiden Hydrochloride
- Biperiden Lactate
- Biphenyldicarboxylate
- Biphenyl Dimethyl Dicarboxylate
- Bisoprolol Fumarate
- Bisoprolol Fumerate
- Bisoprolol Hemifumarate
- Biuret
- Bivalirudin
- Bleomycin Hydrochloride
- Bleomycin Sulfate
- Blonanserin
- Bortezomib
- Bosentan
- Bosentan Monmonohydrateohydrate
- Bosutinib
- Brimonidine Tartrate
- Brinzolamide
- Brodimoprim
- Bromaminic Acid
- Bromfenac Monosodium Salt Sesquihydrate
- Bromfenac Sodium
- Bromhexine Hydrochloride
- Bromoacetonitrile
- Bromocriptine Mesylate
- Brompheniramine Maleate
- Buclizine
- Buclizine Dihydrochloride
- Budesonide
- Buflomedil Hydrochloride
- Bumetanide
- Bunitrolol
- Bupivacaine Base
- Buserelin
- Buserelin Acetate
- Buspirone
- Buspirone Hydrochloride
- Busulfan
- Butafosfan
- Butamirate citrate
- Butenafine hydrochloride
- Butizide
- Butoconazole
- Butopiprine
- Butorphanol Tartrate
- Butylamine
- Butylated hydroxyanisole
- Cabazitaxel
- Cabergoline
- Cabozantinib
- Cadazolid
- Caffeine
- Caffeine anhydrous
- Calamine
- Calcifediol
- Calcipotriol
- Calcipotriol anhydrous
- Calcipotriol monohydrate
- Calcitonin
- Calcitonin Acetate
- Calcitonin salmon
- Calcitriol
- Calcium acetate
- Calcium ascorbate
- Calcium ascorbate dihydrate
- Calcium folinate
- Calcium fructoborate
- Calcium-glubionate
- Calcium hydrogenphosphate dihydrate
- Calcium lactate
- Calcium levofolinate
- Calcium Levulinate
- Calcium Pantothenate
- Calcium Pidolate
- Calcium polycarbophil
- Calcium Sennosides
- Camostat mesilate
- Camptothecin
- Camylofin
- Canagliflozin
- Canagliflozin hemihydrate
- Candesartan
- Cangrelor
- Canrenone
- Canthaxanthin
- Capecitabine
- Capecitabine Hydrochloride
- Capreomycin Sulfate
- Capsaicin
- Captopril
- Carbadox
- Carbaica
- Carbamazepine
- Carbamazepine Bromine
- Carbasalate calcium
- Carbazochrome
- Carbazochrome sodium sulfonate
- Carbenicillin disodium
- Carbetocin
- Carbidopa
- Carbimazole
- Carbinoxamine
- Carbinoxamine Maleate
- Carbocistein
- Carbocysteine
- Carbon disulphide
- Carboplatin
- Carboprost tromethamine
- Carboxymethylcellulose sodium salt
- Carfilzomib
- Carisoprodol
- Carnosic acid
- Caronic anhydride
- Carperitide
- Carpipramine
- Carprofen
- Carteolol
- Carteolol Hydrochloride
- Carvedilol
- Carvedilol hydrochloride
- Carvedilol Phosphate
- Casein
- Caspofungin
- Caspofungin acetate
- Cefaclor
- Cefadroxil
- Cefadroxil Monohydrate
- Cefalonium
- Cefalotin sodium
- Cefamandole nafate
- Cefathiamidine
- Cefatrizine
- Cefazolin
- Cefazolin sodium salt
- Cefcapene
- Cefcapene pivoxil
- Cefcapene pivoxil hydrochloride
- Cefdinir
- Cefditoren
- Cefditoren pivoxil
- Cefditoren sodium
- Cefepime
- Cefepime hydrochloride
- Cefetamet pivoxil hydrochloride
- Cefetamet pivoxyl
- Cefixime
- Cefixime Trihydrate
- Cefmenoxime hydrochloride
- Cefmetazole
- Cefmetazole Acid
- Cefmetazole sodium
- Cefminox sodium
- Cefodizime Acid
- Cefodizime sodium
- Cefonicid sodium
- Cefoperazone
- Cefoperazone sodium
- Cefoselis sulfate
- Cefotaxime
- Cefotaxime Sodium
- Cefotetan
- Cefotetan disodium
- Cefotiam
- Cefotiam Hexetil
- Cefotiam hydrochloride
- Cefoxitin
- Cefoxitin sodium
- Cefozopran
- Cefpimizole
- Cefpiramide sodium
- Cefpirome
- Cefpirome sulfate
- Cefpodoxime
- Cefpodoxime proxetil
- Cefprozil
- Cefquinome sulfate
- Cefradine
- Cefsulodin sodium
- Cefsulodine sodium
- Ceftazidime
- Ceftazidime dihydrochloride
- Ceftazidime Pentahydrate
- Cefteram
- Ceftezole
- Ceftezole sodium
- Ceftibuten
- Ceftibuten dihydrate
- Ceftiofur
- Ceftiofur hydrochloride
- Ceftiofur Sodium
- Ceftizoxime
- Ceftizoxime alapivoxil
- Ceftizoxime sodium
- Ceftriaxone
- Ceftriaxone disodium salt hemi(heptahydrate)
- Ceftriaxone sodium
- Cefuroxime Axetil
- Cefuroxime sodium
- Celecoxib
- Celiprolol hydrochloride
- Cephalexin
- Cephalomannine
- Cephalothin
- Cephapirin benzathine
- Cephaprin
- Cetilistat
- Cetirizine
- Cetirizine hydrochloride
- Cetrimide
- Cetrorelix
- Cetrorelix acetate
- Cetylpyridinium bromide
- Cetylpyridinium Chloride
- Cetylpyridinium chloride monohydrate
- Cevimeline
- Cevimeline Hydrochloride
- Chenodeoxycholic acid
- Chitosan
- Chlorambucil
- Chloramine B
- Chloranil
- Chlorantraniliprole
- Chlorcyclizine Hydrochloride
- Chlordiazepoxide
- Chlordiazepoxide hydrochloride
- Chlorhexidine acetate
- Chlorhexidine Base
- Chlorhexidine Gluconate
- Chlorhexidine hydrochloride
- Chlormadinone acetate
- Chloroacetone
- Chloroamphenicol stearate
- Chlorobutanol
- Chlorobutanol (anhydrous)
- CHLOROBUTANOL HEMIHYDRATE
- Chlorogenic acid
- Chloropyramine
- Chloropyramine hydrochloride
- Chloroquine Phosphate
- Chloroquine sulfate
- Chlorothiazide
- Chlorphenesin
- Chlorpheniramine
- Chlorpheniramine Maleate
- CHLORPHENIRAMINE TANNATE
- Chlorpromazine
- Chlorpromazine hydrochloride
- Chlorpropamide
- Chlorprothixene hydrochloride
- Chlortalidone
- Chlortetracycline
- Chlortetracycline hydrochloride
- Chlorzoxazone
- Cholecalciferol
- Cholesterol
- Choline bitartrate
- Choline chloride
- Choline Fenofibrate
- Choline glycerophosphate
- Choline Salicylate
- Choline Theophyllinate
- Chondroitin
- Chondroitin sulfate
- Chorionic gonadotrophin
- Chromium picolinate
- Chromium Polynicotinate
- Chymotrypsin
- Cibenzoline succinate
- Ciclesonide
- Ciclopirox
- Ciclopirox ethanolamine
- Ciclopirox olamine
- Cilansetron
- Cilastatin
- Cilastatin sodium
- Cilazapril
- Cilnidipine
- Cilostazol
- Cimetidine
- Cimetropium bromide
- Cinacalcet
- Cinacalcet Hydrochloride
- Cinchonidine
- Cinchonine
- Cinchophen
- Cinepazide Maleate
- Cinitapride Hydrogen Tartrate
- Cinnarizine
- Cinoxacin
- Ciprofibrate
- Ciprofloxacin
- Ciprofloxacin hydrochloride
- Ciprofloxacin Hydrochloride Hydrate
- Ciprofloxacin lactate
- Cisatracurium Besylate
- Cisplatin
- Citicoline Sodium
- Clarithromycin
- Clarithromycin Lactobionate
- Clemastine Fumarate
- Clevidipine Butyrate
- Cleviprex
- Clidinium Bromide
- Clindamycin
- Clindamycin Hydrochloride
- Clindamycin Palmitate Hydrochloride
- Clindamycin Phosphate
- Clinofibrate
- Clobazam
- Clobetasol Propionate
- Clobetasone Butyrate
- Clocapramine
- Clocortolone
- Clocortolone Pivalate
- Clofarabine
- Clofazimine
- Clomifene
- Clomifene Citrate
- Clomipramine
- Clomipramine Hydrochloride
- Clonidine
- Clonidine Hydrochloride
- Clopamide
- Cloperastine
- Cloperastine Hydrochloride
- Clopidogrel
- Clopidogrel Bisulfate
- Clopidogrel Hydrobromide
- Clopidogrel Hydrochloride
- Clopidogrel Hydrogen Sulfate
- Clopidol
- Cloprostenol Sodium
- Clorazepate Dipotassium
- Clorprenaline Hydrochloride
- Clorsulon
- Closantel Base
- Clotiazepam
- Clotrimazole
- Cloxacillin Benzathine
- Cloxacillin Sodium
- Cloxazolam .
- Clozapine
- Cobamamide
- Cobicistat
- Coenzyme
- Colchicine
- Colesevelam
- Colesevelam Hydrochloride
- Colestilan
- Colestipol hydrochloride
- Colistimethate Sodium
- Colistin
- Colistin Sulfate
- Collagen
- Conivaptan
- Conivaptan Hydrochloride
- Conjugated Estrogens
- Copovidone
- Copper Gluconate
- Cordycepin
- Cortisone
- Cortisone Acetate
- Coumarin
- Creatinine
- Crizotinib
- Crotamiton
- Curcumin
- Cyanocobalamin
- Cyanotemozolomide
- Cyclandelate
- Cyclizine
- Cyclizine Hydrochloride
- Cyclobenzaprine
- Cyclobenzaprine Hydrochloride
- Cyclopentene Oxide
- Cyclopentolate
- Cyclopentolate Hydrochloride
- Cyclophosphamide
- Cyclophosphamide Monohydrate
- Cycloserine
- Cyclosporin A
- Cyclosporine
- Cyfluthrin
- Cymiazole
- Cypermethrin
- Cyproheptadine
- Cyproheptadine Hydrochloride
- Cyproterone Acetate
- Cyromazine
- Cystine
- Cytarabine
- Cytarabine Hydrochloride
- Cytidylic Acid
- Cytisine
- Cytochrome C
- Cytosine
- (S)-(+)-2-Phenylpropionic Acid
- Dabrafenib Intermediate
- Dacarbazine
- Daclatasvir
- Daclatasvir Dihydrochloride
- Dactinomycin
- Dalbavancin
- Dalfampridine
- Dalfopristin
- Dalteparin
- Dalteparin Sodium
- D-Amphetamine Base
- D-Amphetamine Saccharate
- Danazol
- Danofloxacin Mesylate
- Dantrolene Sodium
- Dapagliflozin
- Dapsone
- Daptomycin
- Darifenacin
- Darifenacin Hydrobromide
- Darunavir
- DARUNAVIR AMORPHOUS
- Darunavir Ethanolate
- Dasatinib
- Dasatinib Monohydrate
- Daunorubicin
- Daunorubicin Hydrochloride
- D-Biotin
- D-Cloprostenol Sodium
- D-Cycloserine
- Deacetylnorgestimate
- Deacetyltaxol
- Decatol
- Decitabine
- Decloxizine
- Decloxizine Hydrochloride
- Decoquinate
- Deferasirox
- Deferiprone
- Deferoxamine Mesylate
- Deflazacort
- Degarelix
- Degarelix Acetate
- Dehydroepiandrosterone
- Dehydroepiandrosterone Enanthate
- Delafloxacin
- Delamanid
- Delavirdine
- Deltaline
- Deltamethrin
- Demeclocycline
- Demeclocycline Hydrochloride
- Denopamine
- Deoxyarbutin
- Deoxyribonuclease
- Depofemin
- Deprodone
- Dersalazine
- Descinolone
- Deserpidine
- Desflurane
- Desipramine Hydrochloride
- Desirudin
- Desloratadine
- Deslorelin
- Desmopressin
- Desmopressin Acetate
- Desogestrel
- Desonide
- Desoximetasone
- Desvenlafaxine
- Desvenlafaxine Fumarate
- Desvenlafaxine succinate
- Detomidine hydrochloride
- Dexamethasone
- Dexamethasone Acetate
- Dexamethasone Isonicotinate
- Dexamethasone Sodium Phosphate
- Dexamethasone Valerate
- Dexbrompheniramine Maleate
- Dexchlorpheniramine
- Dexchlorpheniramine Maleate
- Dexketoprofen trometamol
- Dexlansoprazole
- Dexmedetomidine
- Dexmedetomidine Hydrochloride
- Dexpramipexole
- Dexrabeprazole Sodium
- Dexrazoxane
- Dexrazoxane Hydrochloride
- Dextran
- Dextromethorphan Hydrobromide
- Dextrose Anhydrous
- Dextrose Monohydrate
- Dezocine
- D-Glucosamine Sulfate
- D-Glucuronolactone
- D-Glutamine
- Diacerein
- Diammonium Glycyrrhizinate
- Diatrizoate Meglumine
- Diatrizoate Sodium
- Diatrizoic Acid
- Diaveridine
- Diaveridine Hydrochloride
- Dibucaine Base
- Dibucaine Hydrochloride
- DI-Calcium Phosphate
- DICHLORALPHENAZONE
- Diclazuril
- Diclofenac
- Diclofenac Diethylamine
- Diclofenac Epolamine
- Diclofenac Potassium
- Diclofenac Sodium
- Dicloxacillin Sodium
- Dicyandiamide
- Dicyclomine Hydrochloride
- Dideoxyadenosine
- Dienogest
- Diethylamine Salicylate
- Diethylcarbamazine Citrate
- Difethialone
- Diflomotecan
- Diflorasone
- Diflorasone Diacetate
- Difloxacin Hydrochloride
- Diflucortolone
- Diflucortolone Valerate
- Diflunisal
- Difluprednate
- Digoxin
- Dihydralazine
- Dihydralazine Mesylate
- Dihydralazine Sulfate
- Dihydroartemisinin
- Dihydrocodeine Hydrogentartrate
- Dihydroergocristine Mesylate
- Dihydroergocryptine Mesylate
- Dihydroergotamine Mesylate
- Dihydroergotoxine Mesylate
- Dihydroquercetin
- Dihydrostreptomycin Sulfate
- Dihydroxyaluminum Sodium Carbonate
- Diloxanide Furoate
- Diltiazem
- Diltiazem hydrochloride
- Dimenhydrinate
- Dimercaptosuccinic acid
- Dimethyl fumarate
- Dimetridazole
- Diminazene
- Diminazene Diaceturate
- Diniconazole
- Dinitolmide
- Dinoprost Tromethamine
- Diosgenin
- Diosmetin
- Diosmin
- Diphemanil Mesylate
- Diphencyprone
- Diphenhydramine
- Diphenhydramine Citrate
- Diphenhydramine Hydrochloride
- Diphenhydramine citrate
- Dipotassium Hydrogen Phosphate Trihydrate
- Diprophylline .
- Dipyridamole
- Diquafosol
- Dirithromycin
- Disodium Monofluorophosphate
- Disopyramide
- Disopyramide Phosphate
- Distigmine Bromide
- Disulfiram
- Dithranol
- Divalproex Sodium
- DL-alpha-Hydroxymethionine Calcium
- DL-Amphetamine Asphartate
- DL-Amphetamine Sulphate
- DL-Cloprostenol Sodium
- DL-Lysine
- DL-Lysine acetylsalicylate
- DL-Octopamine hydrochloride
- DL-O-Phosphorylserine
- DL-Selenomethionine
- D-Methyl Phenidate Hydrochloride
- D-Norpseudoe Phedrine Hydrochloride
- Dobutamine Hydrochloride
- Docetaxel
- Docetaxel anhydrous
- Docetaxel trihydrate
- Dofetilide
- Dolasetron mesylate
- Dolasteron
- Dolutegravir
- Dolutegravir Sodium
- Domiphen Bromide
- Domperidone
- Domperidone Maleate
- Donepezil Base
- Donepezil Hydrochloride
- Dopamine Hydrochloride
- Doramectin
- Doripenem
- Doripenem Monohydrate
- Dorzolamide
- Dorzolamide Hydrochloride
- Dothiepin
- Doxapram
- Doxapram Hydrochloride Monohydrate
- Doxazosin
- Doxazosin Mesylate
- Doxepin
- Doxepin Hydrochloride
- Doxercalciferol
- Doxibetasol
- Doxifluridine
- Doxofylline
- Doxorubicin
- Doxorubicin Hydrochloride
- Doxycycline
- Doxycycline Hyclate
- Doxycycline hydrochloride
- Doxycycline Monohydrate
- Doxylamine
- Doxylamine Succinate
- Drocinonide Phosphate Potassium
- Dromostanolone Propionate
- Dronabinol
- Dronedarone
- Dronedarone Hydrochloride
- Droperidol
- Drospirenone
- Drotaverine
- Drotaverine Hydrochloride
- Droxidopa
- Duloxetine
- Duloxetine Hydrochloride
- Dutogliptin
- Dybenal
- Dyclonine Hydrochloride
- Dynorphin A
- Ebastine
- Ebastine Fumarate
- Ecabet Sodium
- Econazole
- Econazole Nitrate
- Edaravone
- Edoxaban
- Edoxaban Tosylate
- Efavirenz
- EFINACONAZOLE
- Eflornithine
- Eflornithine Hydrochloride
- Eflornithine Hydrochloride Hydrate
- Efonidipine .
- Elastase
- Elcatonin
- Elcatonin Acetate
- Eldecalcitol
- Eledoisin
- Eletriptan
- Eletriptan Hydrobromide
- Eletriptan Hydrobromide Monohydrate
- Eliglustat
- Eluxadoline
- Elvitegravir
- Emedastine Difumarate
- Emetine Hydrochloride
- Empagliflozin
- Emtricitabine
- Enalapril
- Enalapril Maleate
- Enalaprilat
- Enfuvirtide
- Enkephalin
- Enoxacin
- Enoxaparin Sodium
- Enoxolone
- Enrofloxacin
- Enrofloxacin Hydrochloride
- Entacapone
- Entecavir
- Entecavir Hydrate
- Entecavir Monohydrate
- Enzalutamide
- Enzastaurin
- Epalrestat
- Eperisone Hydrochloride
- Epiandrosterone
- Epiandrosterone Acetate
- Epibrassinolide
- Epiestradiol
- Epinastine Hydrobromide
- Epinephrine
- Epinephrine Bitartrate
- Epirubicin
- Epirubicin Hydrochloride
- Epitiostanol
- Eplerenone
- Epocholeone
- Epoprostenol Sodium
- Epothilone B
- Eprazinone Dihydrochloride
- Eprinomectin
- Eprosartan
- Eprosartan Mesylate
- Eptaplatin
- Eptifibatide
- Eptifibatide Acetate
- Erdosteine
- Ergosterol
- Ergotamine Tartrate
- Eribulin
- Erlotinib
- Erlotinib Hydrochloride
- Ertapenem
- Ertapenem Disodium
- Ertapenem Sodium
- Erythritol
- Erythromycin
- Erythromycin Estolate
- Erythromycin Ethylsuccinate
- Erythromycin Lactobionate
- Erythromycin Oxime
- Erythromycin Phosphate
- Erythromycin Propionate
- Erythromycin Stearate
- Erythromycin Thiocyanate
- Eslicarbazepine
- Eslicarbazepine Acetate
- Esmolol Hydrochloride
- Esomeprazole
- Esomeprazole Magnesium
- Esomeprazole Magnesium Dihydrate
- Esomeprazole Magnesium Trihydrate
- Esomeprazole Sodium
- Estazolam
- Estradiol
- Estradiol Benzoate
- Estradiol Benzoate Butyrate
- Estradiol Cypionate
- Estradiol Dipropionate
- Estradiol Enanthate
- Estradiol Hemihydrate
- Estradiol Hexahydrobenzoate
- Estramustine Phosphate Sodium
- Estriol
- Estrone
- Eszopiclone
- Etafedrine Hydrochloride
- Etamiphylline Camsylate
- Etamsylate
- Etanercept
- Ethacridine Lactate
- Ethacrynate Sodium
- Ethacrynic Acid
- Ethanol
- Ethisterone
- Ethopabate
- Ethosuximide
- Ethyl Icosapentate
- Ethyl Loflazepate
- Ethyl Vanillin
- Ethylestrenol
- Ethylmorphine Hydrochloride
- Ethylparaben
- Ethynodiol Diacetate
- Ethynyl Estradiol
- Etidronate Disodium
- Etidronate Sodium
- Etiproston
- Etocrylene
- Etodolac
- Etofenamate
- Etofylline
- Etofylline Clofibrate
- Etofylline Nicotinate
- Etomidate
- Etonogestrel
- Etoposide
- Etoricoxib
- Etravirine
- Everolimus
- Exemestane
- Exenatide
- Exenatide Acetate
- Ezetimibe
- Ezogabine
- Faldaprevir
- Famciclovir
- Famotidine
- Faropenem Sodium
- Faropenem Sodium Hemipentahydrate
- Fasudil Hydrochloride
- Favipiravir
- Febantel
- Febuxostat
- Felbamate
- Felbinac
- Felodipine
- Felypressin
- Felypressin Acetate
- Fenazox
- Fenbendazole
- Fendizoic Acid
- Fenofibrate
- Fenofibric Acid
- Fenoprofen Calcium
- Fenoterol
- Fenoterol Hydrobromide
- Fenoxazoline Hydrochloride
- Fenpiverinium Bromide
- Fenproporex Hydrochloride
- Fenpyroximate
- Fenspiride
- Fenspiride Hydrochloride
- Fenticonazole Nitrate
- Ferric Citrate
- Ferrous Ascorbate
- Fertirelin
- Fertirelin Acetate
- Fesoterodine
- Fesoterodine Fumarate
- Fexofenadine
- Fexofenadine Hydrochloride
- Fidaxomicin
- Fimasartan
- Finasteride
- Fingolimod
- Fingolimod Hydrochloride
- Fipronil
- Flavopiridol Hydrochloride
- Flavoxate
- Flavoxate Hydrochloride
- Flecainide
- FLECAINIDE ACETATE
- Fleroxacin
- Flibanserin
- Flibanserin Hydrochloride
- Flofenicol
- Flomoxef
- Flonicamid
- Florfenicol
- Floxuridine
- Fluazuron
- Flubendazole
- Flucloxacillin Magnesium
- Flucloxacillin Sodium
- Fluconazole
- Fludarabine
- Fludarabine Phosphate
- Fludiazepam
- Fludioxonil
- Fludrocortisone
- Fludrocortisone Acetate
- Flufenamic Acid
- Flugestone
- Fluindione
- Flumequine
- Flumequine Sodium
- Flumethasone
- Flumethasone Acid
- Flumethrin
- Flumizole
- Flunarizine
- Flunarizine Dihydrochloride
- Flunisolide
- Flunixin Meglumine
- Fluocinolone Acetonide
- Fluocinonide
- Fluocortolone
- Fluorescein Sodium
- Fluorogesterone Acetate
- Fluorometholone
- Fluorometholone Acetate
- Fluoxetine
- Fluoxetine Hydrochloride
- Flupenthixol Dihydrochloride
- Fluphenazine Decanoate
- Fluphenazine Hydrochloride
- Flupirtine
- Flupirtine Maleate
- Fluprednidene
- Fluprednisolone
- FLUPREDNISOLONE ACETATE
- Flurazepam Dihydrochloride
- Flurbiprofen
- Flurbiprofen Axetil
- Flusilazole
- Flutamide
- Fluticasone
- Fluticasone Furoate
- Fluticasone Propionate
- Flutriafol
- Flutrimazole
- Fluvalinate
- Fluvastatin
- Fluvastatin Sodium
- Fluvoxamine
- Fluvoxamine Maleate
- Folcisteine
- Folic Acid
- Folinic Acid
- Follicle Stimulating Hormone
- Fomepizole
- Fondaparinux Sodium
- Formoterol Fumarate
- Formoterol Fumarate Dihydrate
- Forskolin
- Fosamprenavir Calcium
- Fosaprepitant
- Fosaprepitant Dimeglumine
- Foscarnet sodium
- Fosfluconazole
- Fosfomycin
- Fosfomycin Calcium
- Fosfomycin Phenylethylamine
- Fosfomycin Sodium
- Fosfomycin Trometamol
- Fosinopril
- Fosinopril Sodium
- Fosphenytoin Sodium
- Fozivudine
- Framycetin Sulphate
- Frovatriptan
- Frovatriptan Succinate
- Fudosteine
- Fulvestrant
- Fupentixol Dihydrochloride
- Furaltadone
- Furaltadone Hydrochloride
- Furazabol
- Furazolidone
- Furbenicillin
- Furosemide
- Fursultiamine
- Fursultimine Hydrochloride
- Fusidate Sodium
- Fusidine
- Gabapentin
- Gabapentin enacarbil
- Gabexate mesylate
- Gadobutrol
- Gadodiamide
- Gadofosveset Trisodium
- Gadopentetate Dimeglumine
- Gadoterate meglumine
- Galanthamine
- Galanthamine hydrobromide
- Gamithromycin
- Ganciclovir
- Ganciclovir Sodium
- Garenoxacin
- Garenoxacin Mesylate
- Gastrodin
- Gefitinib
- Geldanamycin
- Gemcitabine
- Gemcitabine hydrochloride
- Gemfibrozil
- Gemifloxacin
- Gemifloxacin Mesylate
- Gentamicin Sulfate
- Gestodene
- Gestonorone
- Gestrinone
- Gimeracil
- Glabridin
- Glatiramer Acetate
- Glibenclamide
- Gliclazide
- Glimepiride
- Glipizide
- Gliquidone
- Glucagon Hydrochloride
- Glucono Delta Lactone
- Glucosamine
- Glucosamine Hydrochloride
- Glucosamine Sulfate
- Glutaric Acid
- Glutathione
- Glycerol Phosphate Calcium Salt
- Glycine
- Glycopyrrolate
- Glycylglycine
- Glycyl-glycyl-glycine
- Glycyl-L-glutamine Monohydrate
- Glycyl-L-tyrosine
- Glycyrrhizic Acid Ammonium Salt
- Gly-Leu
- Gonadorelin
- Gonadorelin Acetate
- Goserelin
- Goserelin Acetate
- Gramine
- Granisetron Base
- Granisetron Hydrochloride
- Griseofulvin
- Growth Hormone Releasing Peptide (GHRP-6)
- Guaifenesin
- Guanethidine Sulfate
- Guanidine Nitrate
- Guanosine
- Halcinonide
- Halobetasol Propionate
- Halofantrine Hydrochloride
- Halofuginone
- Halometasone
- Haloperidol
- Haloperidol Decanoate
- Halquinol
- Heparin Sodium
- Heparinoid
- Heptaminol
- Heptaminol hydrochloride
- Hesperetin
- Hesperidin
- Hesperidin methylchalcone
- Hexaconazole
- Hexarelin
- Hexetidine
- Hexythiazox
- Histrelin
- Histrelin acetate
- Homatropine hydrobromide
- Homatropine methylbromide
- Homidium Chloride
- Hordenine
- Human GLP-1 (7-37)
- Huperzine A
- Hyaluronic acid
- Hyaluronidase
- Hydralazine
- Hydralazine hydrochloride
- Hydrazine acetate
- Hydrochlorothiazide
- Hydrocortisone
- Hydrocortisone aceponate
- Hydrocortisone acetate
- Hydrocortisone butyrate
- Hydrocortisone hemisuccinate
- Hydrocortisone sodium phosphate
- Hydrocortisone Sodium Succinate
- Hydrocortisone valerate
- Hydromadinone
- Hydroquinone
- Hydrotalcite
- Hydrotriamcinolone Acetate
- Hydroxocobalamin .
- Hydroxocobalamin acetate
- Hydroxocobalamin Hydrochloride
- Hydroxocobalamin Sulphate
- Hydroxyamphetamine Hydrobromide
- Hydroxychloroquine sulfate
- Hydroxyethyl Salicylate
- Hydroxyurea
- Hydroxyzine hydrochloride
- Hydroxyzine Pamoate
- Hygromycin B
- Hyoscine Butylbromide
- Hyoscyamine sulphate
- Hyoscyamine sulphate hydrate
- Hypoxanthine
- Ibandronate Sodium
- Ibudilast
- Ibuprofen
- Ibuprofen Arginine
- Ibutilide fumarate
- Icatibant
- Icatibant acetate
- Idarubicin
- Idarubicin hydrochloride
- Idebenone
- Idelalisib
- Ifenprodil
- Ifenprodil tartrate
- Ifosfamide
- Ilaprazole
- Iloperidone
- Iloprost
- Imatinib mesylate
- Imazamox
- Imidacloprid
- Imidafenacin
- Imidapril
- Imidapril Hydrochloride
- Imidazole
- Imidocarb dipropionate
- Imipenem
- Imipramine hydrochloride
- IMIPRAMINE PAMOATE
- Imiquimod
- Indacaterol
- Indacaterol maleate
- Indapamide
- Indinavir
- Indinavir sulfate
- Indometacin
- Indomethacin
- Indomethacin sodium
- Infliximab
- Ingenol Mebutate
- Inosine
- Inositol
- Intedanib
- Iodixanol
- Iohexol
- Iopamidol
- Iotrolan
- Ioversol
- Ipragliflozin
- Ipragliflozin L-Proline
- Ipratropium Bromide
- Ipriflavone
- Irbesartan
- Irinotecan
- Irinotecan hydrochloride
- Irinotecan hydrochloride trihydrate
- Iron lll Hydroxide Poly Maltose complex
- Iron sorbitol
- Iron Sucrose
- Iron-dextran
- Irsogladine
- Isavuconazole
- Isavuconazonium sulfate
- Isepamicin sulfate
- Isocarboxazid
- Isoconazole nitrate
- Isoflavone
- Isoflupredone
- Isoflupredone acetate
- Isoflurane
- Isometamidium chloride
- Isometamidium Chloride Hydrochloride
- Isometheptene mucate
- Isoniazid
- Isophosphamide
- Isoprenaline hydrochloride
- Isoprenaline sulphate
- Isoprinosine
- Isopropamide Bromide
- Isopropamide iodide
- Isopropyl Methylphenol
- Isopropyl unoprostone
- Isoproterenol
- Isosorbide 5-mononitrate
- Isosorbide dinitrate
- Isosulfan Blue
- Isothipendyl
- Isotretinoin
- Isoxadifen ethyl ester
- Isoxaflutole
- Isoxsuprine hydrochloride
- Istradefylline
- Itopride
- Itopride hydrochloride
- Itraconazole
- Ivabradine
- Ivabradine hydrochloride
- Ivacaftor
- Ivermectin
- Ixabepilone
- Josamycin
- Julolidine
- Kanamycin
- Kanamycin sulfate
- Ketazolam .
- Ketoconazole
- Ketoketal
- Ketoprofen
- Ketoprofen lysinate salt
- Ketorolac
- Ketorolac Tromethamine
- Ketotifen
- Ketotifen Fumarate
- Kitasamycin
- Kitasamycin tartrate
- Kojic acid
- L(-)-Glutathione
- L(+)-Ascorbic acid
- L-(+)-Glutamic acid hydrochloride
- LABETALOL
- Labetalol Hydrochloride
- Labetalone hydrochloride
- Lacidipine
- Lacosamide
- Lactic Acid
- Lactose
- Lafutidine
- Lagociclovir .
- Lamivudine
- Lamivudine Salicylate
- Lamotrigine
- Lanoconazole
- Lanolin
- Lanreotide
- Lanreotide acetate
- Nutraceuticals .
- Chemical Intermediates
- (1R,2R) 1,2 Cyclohexanedimethanol
- Pharmaceutical excipients
- (R)-(+) Glycidol
- N Lodosuccinimide
- (1alpha)-17-(Acetyloxy)-6-chloro-1-(chloromethyl)
- (1R,2R,3S,5R) (-) 2,3 Pinanediol
- (2-Fluorobenzyl) Hydrazine
- (3,4-Dimethoxyphenyl) Acetic Acid
- (3R) 3 Amino 1 Butanol
- Bromomethyl Cyclopropane
- (Phenylazo) Malonitrile
- (1-Benzyl-4-piperidyl)methanol
- (1-Ethyl-2-Phenoxy-Propyl)- Hydrazine L Dibenzoly
- (1-Hydroxycyclohexyl)(4-hydroxyphenyl)acetonitril
- (1-Hydroxycyclopentyl)Phenylacetic Acid
- (1-Methyl-4-piperidinyl)[3-[2-(3-chlorophenyl)eth
- (1-Methylpiperidin-4-Yl) 2-Hydroxy-2,2-Di(Phenyl)
- (1R,1'R)-2,2'-(3,11-Dioxo-4,10-dioxatridecamethyl
- (1R,2R,3S,5R)-(-)-2,3-Pinanediol
- (1R,2R)-1-[(3,4-Dimethoxyphenyl)methyl]-2-[3-(ter
- (1R,2R)-1,2-Cyclohexanedicarboxylic acid
- (1R,2R)-1,2-Cyclohexanedimethanol
- (1R,2R)-2-(3,4-difluorophenyl)cyclopropanecarboxy
- (1R,2R)-2-Amino-1-(4-nitrophenyl)propane-1,3-diol
- (1R,2R)-(-)-1,2-Diaminocyclohexane
- (1R,2R)-(+)-1,2-Diphenylethylenediamine
- (1R,2R)-(-)-N-(4-Toluenesulfonyl)-1,2-diphenyleth
- (1R,2S,5R)-7-Oxo-6-(phenylmethoxy)-1,6-diazabicyc
- (1R,2S,5R)-Menthyl-(2R,5S)-5-(4-amino-2-oxo-2H-py
- (1R,2S)-1-Amino-2-ethenylcyclopropanecarboxylic a
- (1R,2S)-1-Amino-2-vinylcyclopropanecarboxylic aci
- (1R,2S)-1-Amino-N-(cyclopropylsulfonyl)-2-ethenyl
- (1R,2S)-1-tert-Butoxycarbonylamino-2-vinylcyclopr
- (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanamine
- (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanamine (2
- (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanaminium
- (1R,2S)-2-Amino-1,2-diphenylethanol
- (1R,2S)-2-Ethenyl-1-[[[(1R,2R,4S)-2-[(5-hexen-1-y
- (1R,2S)-2-Fluorocyclopropylamine tosylate
- (1R,2S)-2-[(Phenylmethoxy)methyl]-3-cyclopenten-1
- (1R,2S)-Ethyl 1-amino-2-vinylcyclopropanecarboxyl
- (1R,2S)-Methyl 1-amino-2-vinylcyclopropanecarboxy
- (1R,2S)-rel-2-(3,4-Difluorophenyl)cyclopropanamin
- (1R,3aR,7aR)-1-((R)-6-hydroxy-6-Methylheptan-2-yl
- (1R)-2-[[6-[2-[(2,6-Dichlorobenzyl)oxy]ethoxy]hex
- (1R,3aR,7aR)-1-((S)-1-hydroxypropan-2-yl)-7a-Meth
- (1R)-(-)-Menthyl glyoxylate hydrate
- (10-Phenylanthracen-9-yl)boronic acid
- (17beta)-13-Ethyl-17-hydroxy-11-methylenegon-4-en-
- (17)-3,3-[1,2-Ethanediylbis(oxy)]-17-hydroxy-19-
- (-)-1-[(4-Chlorophenyl)phenylmethyl]piperazine
- (-)-4-(4-Dimethylamino)-1-(4-fluorophenyl)-1-(hyd
- ((5-Thiazolyl)methyl)-(4-nitrophenyl)carbonate
- (+)-2,3-O-Isopropylidene-L-threitol
- (+)-5-Iodo-2'-deoxyuridine
- (+)-(3aR,4R,5r,6aS)-Hexahydro-5-hydroxy-4-[(1E,3R
- (+)-(6R)-2,6-Diamino-4,5,6,7-tetrahydrobenzothiaz
- (+%2f-)1-(1-Naphthyl)ethylamine
- (+%2f-)-2,2-Dimethyl-1,3-dioxolane-4-methanol
- (+%2f-)-4-[4-(diMethylaMino)-1-(4-fluorophenyl)-1
- (+%2f-)-trans-1,2-Diaminocyclohexane
- ()-1,2-Diphenyl-1-[4-[2-(dimethylamino)ethoxy]ph
- (1 R)-3-Chloro-1-phenyl-propan-1-ol
- (1R,2R,4R)-2-[(5-Hexen-1-yl methylamino)carbonyl]-
- (1R,2R,4S)-2-[(5-Hexen-1-yl methylamino)carbonyl]-
- (1R,2S)-1-[[(1,1-Dimethylethoxy)carbonyl]amino]-2-
- (1R,2S)-2-Amino-1-(4-hydroxyphenyl)propan-1-ol
- (1 R)-N-Benzyl-1-(1-Naphthyl)Ethylamine Hydrochlor
- (-)-4-Chlorobenzhydrylamine
- (-)-Corey aldehyde benzoate
- (-)-Corey lactone benzoate
- (-)-Corey lactone 4-phenyl benzoate alcohol
- (-)-Corey lactone diol
- (+)-Di-1,4-toluoyl-D-tartaric acid
- (+)-Dibenzoyl-D-tartaric acid
- (+)-Dibenzoyl-D-tartaric acid monohydrate
- (-)-Dibenzoyl-L-tartaric acid monohydrate
- (-)-Diethyl D-tartrate
- (-)-Diisopinocampheyl chloroborane
- (+)-Diisopinocampheyl chloroborane
- (-)-Di-p-toluoyl-L-tartaric acid
- (+)-Methyl (S)-3-hydroxyvalerate
- (-)-O-Acetyl-D-mandelic acid
- (+)-O,O-Di-p-toluoyl-D-Tartaric acid Monohydrate
- (1R-2R)-2-(3,4-diflrorophenyl)cyclopropanamine(2R)
- (1R-2R)-2-(3,4-diflrorophenyl)cyclopropanamine
- (1R-2R)-2-(3,4-diflrorophenyl)cyclopropanamine(R)-
- (1R-2R)-2-(diflrorophenyl)cyclopropanecarboxamide
- (1R-2R)-2-(diflrorophenyl)cyclopropanamine(2R,3R)-
- (1R,3aR,7aR)-1-((S)-1-hydroxypropan-2-yl)-7a-Methy
- (1R,3R)-1-(1,3-Benzodioxol-5-yl)-2-(chloroacetyl)-
- (1R,3S,4S)-3-(6-Bromo-1H-benzimidazol-2-yl)-2-azab
- (1R,3s,5S)-3-(3-Isopropyl-5-methyl-4H-1,2,4-triazo
- (1R,3S,4S)-3-[6-(4,4,5,5-Tetramethyl-1,3,2-dioxabo
- (1R,3S,4S)-N-Boc-2-azabicyclo[2.2.1]heptane-3-carb
- (1R,4R,5R)-3-Oxo-2-oxabicyclo[2.2.1]heptane-5-carb
- (1R,4S)-2-Azabicyclo[2.2.1]hept-5-en-3-one
- (1R,4S)-4-Aminocyclopentene-1-methanol hydrochlori
- (1R,5S)-5-(Dimethylphenylsilyl)-2-(hydroxymethyl)-
- (1R,6R)-2,8-Diazabicyclo[4,3,0]nonane
- (1S,2S,3R,4R)-methyl 3-((R)-1-acetamido-2-ethylbut
- (1S,2S,3R,5S)-(+)-2,3-Pinanediol
- (1S,2S,3S,4R)-3-[(1S)-1-Amino-2-ethyl butyl]-4-[[(
- (1S,2S,3S,5S)-5-(2-Amino-6-(benzyloxy)-9H-purin-9-
- (1S,2S,3S,5S)-5-[2-[[(4-Methoxyphenyl)diphenylmeth
- (1S,2S)-(1-Benzyl-3-chloro-2-hydroxypropyl)carbami
- (1S,2S)-(-)-1,2-Diphenyl-1,2-ethanediamine
- (1S,3aR,6aS)-(2S)-2-Cyclohexyl-N-(2-pyrazinylcarbo
- (1S,3aR,6aS)-Octahydrocyclopenta[c]pyrrole-1-carbo
- (1S,3S,5S)-2-Azabicyclo[3.1.0]hexane-3-carboxamide
- (1S,3S,5S)-2-Azabicyclo[3,3,0]octane-3-carboxylic
- (1S,4R)-4-(2-Amino-6-chloro-9H-purin-9-yl)-2-cyclo
- (1S,4R)-4-[[(1,1-Dimethylethoxy)carbonyl]amino]-2-
- (1S,4R)-4-Amino-2-cyclopentene-1-carboxylic Acid M
- (1S,4R)-(4-Aminocyclopent-2-enyl)methanol hydrochl
- (1S,4R)-cis-4-Acetoxy-2-cyclopenten-1-ol
- (1S,5R,6S)-Ethyl 5-(pentan-3-yl-oxy)-7-oxa-bicyclo
- (1S,5R)-1-Phenyl-3-oxabicyclo[3.1.0]hexan-2-one
- (1S)-1,5-Anhydro-1-C-[3-(benzo[b]thien-2-ylmethyl)
- (1S)-1,5-Anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)
- (1S)-1-Phenyl-1,2,3,4-tetrahydroisoquinoline
- (1S)-4,5-dimethoxy-1-aminomethyl-benzocyclobutane
- (1S)-4,5-Dimethoxy-1-[(methylamino)methyl]benzocyc
- (1S-cis)-4-Amino-2-cyclopentene-1-methanol
- (1S-cis)-4-Amino-2-cyclopentene-1-methanol D-hydro
- (2,2-Dimethoxyethoxy)-Benzene
- (2b,3a,5a,16b,17b)-2-(4-Morpholinyl)-16-(1-pyrroli
- (2b,3a,5a,16b,17b)-17-Acetoxy-3-hydroxy-2-(4-morph
- (2-Bromo-1,1-difluoroethyl)benzene
- (2-Chlorophenyl)Acetaldehyde
- (2E,4R)-5-[1,1'-Biphenyl]-4-yl-4-[[(1,1-dimethylet
- (2E)-3-(5-Nitro-1-cyclohexen-1-yl)-2-propenoic aci
- (2E)-3-[(4S)-2,2-Dimethyl-1,3-dioxolan-4-yl]-2-met
- (2-Fluorobenzyl)hydrazine
- (2-Formamido-1,3-thiazol-4-yl)glyoxylic acid
- (2-Methyl-2-Phenyl-Propyl) Acetate
- (2-Methyl-5-nitrophenyl)guanidine nitrate
- (2r,3s)-benzyloxycarbonylamino no-2-hydroxy-1-(N-i
- (2'R)-2'-Deoxy-2'-fluoro-2'-methyluridine
- (2'R)-2'-Deoxy-2'-fluoro-2'-methyluridine 3',5'-di
- (2R,3R)-1-(Dimethylamino)-3-(3-methoxyphenyl)-2-me
- (2R,3R)-2-(2,4-difluorophenyl)-3-(tetrahydro-2H-py
- (2R,3R)-2-(2,4-Difluorophenyl)-1-(1H-1,2,4-triazol
- (2R,3R)-2,3-Bis[(4-methylbenzoyl)oxy]butanedioic a
- (2R,3S)-2-(2,4-Difluorophenyl)-3-methyl-[(1H-1,2,4
- (2R,3S)-2-(4-Ethyl-2,3-dioxo-1-piperazinecarboxami
- (2R,3S)-2-[(1R)-1-[3,5-Bis(trifluoromethyl)phenyl]
- (2R,3S)-3-(tert-Butoxycarbonyl)amino-1,2-epoxy-4-p
- (2R)-1-(2,4-Difluorophenyl)-2-[(tetrahydro-2H-pyra
- (2R)-2-[(4-Ethyl-2,3-dioxopiperazinyl)carbonylamin
- (2R)-2-Deoxy-2-fluoro-2-methyl-D-erythropentonic a
- (2R)-4-Benzyl-2-[(1R)-1-[3,5-bis(trifluoromethyl)p
- (2'R)-N-Benzoyl-2'-deoxy-2'-fluoro-2'-methylcytidi
- (2R)-rel-6-Fluoro-3,4-dihydro-2-(2R)-2-oxiranyl-2H
- (2R)-rel-6-Fluoro-3,4-dihydro-2-[(2S)-2-oxiranyl]-
- (2R,3S 2S,3R)-2-(2,4-Difluorophenyl)-3-(5-fluoro-4
- (2R,3S,4R,5R)-2-(hydroxyMethyl)-5-(6-((2-(Methylth
- (2R,4R)-1-[(2S)-5-[[Imino(nitroamino)methyl]amino]
- (2R,4S,5R)-3-Benzoyl-2-(4-methoxyphenyl)-4-phenyl-
- (2R,4S)-4-Amino-5-(biphenyl-4-yl)-2-methylpentanoi
- (2R,4S)-5-(Biphenyl-4-yl)-4-[(tert-butoxycarbonyl)
- (2R,5R)-1,6-Diphenyl-2,5-hexanediamine
- (2R,5R)-1,6-Diphenyl-2,5-hexanediamine hydrochlori
- (2R,5R)-5-Hydroxy-1,3-oxathiolane-2-carboxylic aci
- (2R-cis)-4-Amino-1-[2-(hydroxymethyl)-1,3-oxathiol
- (2S,2'S)-2,2'-([1,1'-Biphenyl]-4,4'-diyldi-1H-imid
- (2S,3aR,7aS)-1H-Octahydroindole-2-carboxylic acid
- (2S,3R)-3-((4R)-2,2-Dimethyldioxolan-4-yl)-2-methy
- (2S,3S,5S)-2-(2,6-Dimethylphenoxyacetyl)amino-3-hy
- (2S,3S,5S)-2-(N-((5-Thiazolyl)methoxyca-rbonyl)ami
- (2S,3S,5S)-5-Amino-2-(N-((5-thiazolyl)-methoxycarb
- (2S,3S,5S)-5-(tert-Butoxycarbonylamino)-2-(N-5-thi
- (2S,3S,5S)-5-tert-Butoxycarbonylamino-2-amino-3-hy
- (2S,3S)-1,2-Epoxy-3-(Boc-amino)-4-phenylbutane
- (2S,3S)-1,2-Epoxy-3-(Cbz-amino)-4-phenylbutane
- (2S,3S)-2-(2,4-Difluorophenyl)-1-(1H-1,2,4-triazol
- (2S,3S)-3-aMino-1-chloro-4-phenylbutan-2-ol hydroc
- (2S,3S)-3-Amino-N-cyclopropyl-2-hydroxyhexanamide
- (2S,4E)-5-Chloro-N,N-dimethyl-2-(1-methylethyl)-4-
- (2S,4R)-2-(Hydroxymethyl)-4-[(methylsulfonyl)oxy]-
- (2S,4S)-1-(tert-butoxycarbonyl)-4-(MethoxyMethyl)p
- (2S,4S)-4-(Acetylthio)-2-(hydroxymethyl)-1-pyrroli
- (2S,4S)-4-Cyano-1,2-pyrrolidinedicarboxylic acid 1
- (2S,4S)-4-(Methoxymethyl)-1,2-pyrrolidinedicarboxy
- (2S,5R)-5-[(Phenylmethoxy)amino]-2-piperidinecarbo
- (2S,5S)-1-(4-(tert-butyl)phenyl)-2,5-bis(4-nitroph
- (2S,5S)-5-methylpyrrolidine-2-carboxylic acid
- (2S,5S)-N-Boc-5-methylpyrrolidine-2-carboxylic aci
- (2S,6R)-6-Amino-2-(2-thienyl)-1,4-thiazepan-5-one
- (2S)-1-[[(2-Amino-1,1-dimethylethyl)amino]acetyl]-
- (2S)-1-(Chloroacetyl)-2-pyrrolidine carbonitrile
- (2S)-1-Methyl-2-pyrrolidone carboxylic acid
- (2S)-2-(2-Oxopyrrolidin-1-yl)butanoic acid
- (2S)-2-Amino-4-methyl-1-[(2R)-2-methyloxiranyl]-1-
- (2S)-2-Cyclohexyl-N-(2-pyrazinylcarbonyl)glycyl-3-
- (2S)-3-Amino-1,2-Propanediol
- (2S)-4-(1,3-Dioxoisoindolin-2-yl)-2-hydroxybutanoi
- (2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidi
- (2S)-5-[(2,5-Dioxo-1-pyrrolidinyl)oxy]-5-oxo-2-[(1
- (2S)-(1-Tetrahydropyramid-2-one)-3-methylbutanoic
- (2S-cis)-2-[(Dimethylamino)carbonyl]-4-mercapto-1-
- (2S-cis)-(+)-2,3-Dihydro-3-hydroxy-2-(4-methoxyphe
- (2S)-Hydroxy(Phenyl)Acetic Acid (1S)-3-(Dimethylam
- (2S)-N-(2,6-Dimethylphenyl)-2-piperidinecarboxamid
- (2S-trans)-3-Amino-2-methyl-4-oxoazetidine-1-sulph
- (2Z)-4-Oxo-4-[3-(trifluoromethyl)-5,6-dihydro-[1,2
- (3-(2-Aminoethyl)-1H-indol-5-yl)-N-methyl methanes
- (3-[5-Cyano-1-(4-fluorophenyl) (1,3-dihydroisobenz
- (3,4-Dimethoxyphenyl)acetic acid
- (3,4-Dimethoxyphenyl)acetonitrile
- (3,5-Diphenylphenyl)boronic acid
- (3alpha,5beta,6E)-6-Ethylidene-3-hydroxy-7-oxochol
- (3alpha,5beta)-6-Ethylidene-3-hydroxy-7-oxocholan-
- (3-Amino-Pyridin-2-Yl)-Methanol
- (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-
- (3aR,4R,5R,6aS)-hexahydro-5-triethylsilane-4-((E)-
- (3aR,4R,5R,6aS)-Hexahydro-4-[(1E,3S)-3-hydroxy-5-p
- (3aR,4R,6S,6aS)-4-[[(1,1-Dimethylethoxy)carbonyl]a
- (3aR,4R,6S,6aS)-4-(tert-butoxycarbonylaMino)-3-(pe
- (3aR,4S,6R,6aS)-6-[7-Amino-5-(propylthio)-3H-1,2,3
- (3aR,4S,6R,6aS)-6-Aminotetrahydro-2,2-dimethyl-4H-
- (3aR,4S,7R,7aS)-rel-Hexahydro-4,7-methano-1H-isoin
- (3aR,7aR)-4'-(1,2-Benzisothiazol-3-yl)octahydrospi
- (3aR,12bR)-rel-5-Chloro-2,3,3a,12b-tetrahydro-2-me
- (3aR,4S,6R,6aS)-6-aminotetrahydro-2,2-dim
- (3aS,4R,5S,6aR)-(+)-Hexahydro-5-hydroxy-4-(hydroxy
- (3aS,4R,6S,6aR)-(6-Amino-2,2-dimethyl-tetrahydrocy
- (3aS,4S,6aR)-Tetrahydro-4-methoxy-furo[3,4-b]furan
- (3aS,6aR,9aR,9bS)-Decahydro-3,6,9-tris(methylene)a
- (3aS,6aR)-Tetrahydro-4-methoxyfuro[3,4-b]furan-2(3
- (3b,5b,15a,16a)-15,16-Dihydro-3,5-dihydroxy-3'H-cy
- (3beta,7alpha,15alpha)-3,7,15-Trihydroxy-androst-5
- (3-Chloropropyl)carbamic acid tert-butyl ester
- (3-Chloropropyl)pyrrolidine hydrochloride
- (3-Dimethylamino)propyltriphenylphosphonium bromid
- (3E)-2,3-Dihydro-3-(methoxyphenylmethylene)-2-oxo-
- (3-Fluoro-4-morpholin-4-ylphenyl)carbamic acid ben
- (3-Fluoro-4'-Pentyl-4-Biphenylyl)Boronic Acid
- (3R,3aR,4S,4aR,7R,8aR,9aR)-7-[(Ethoxycarbonyl)amin
- (3R,3aS,4S,4aS,7R,9aR)-1,3,3a,4,4a,5,6,7,8,9a-Deca
- (3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-ol
- (3R,4R)-1-Benzyl-N,4-dimethylpiperidin-3-amine
- (3R,4R)-3,4-Dimethyl-4-(3-hydroxyphenyl)piperidine
- (3R,4R)-N,4-Dimethyl-1-(phenyl methyl)-3-piperidin
- (3R,4R)-N,4-Dimethyl-1-(phenylmethyl)-3-piperidina
- (3R,4S)-1-(4-Fluorophenyl)-3-[(3S)-3-(4-fluorophen
- (3R,4S)-1-Benzoyl-3-(1-ethoxyethoxy)-4-phenyl-2-az
- (3R,4S)-1-Benzoyl-4-phenyl-3-[(triethylsilyl)oxy]-
- (3R,4S)-3-Hydroxy-4-phenyl-2-azetidinone
- (3R,4S)-4-[4-(Benzyloxy)phenyl]-1-(4-fluorophenyl)
- (3R,4S)-4-Phenyl-3-[(Triethylsilyl)Oxy]-2-Azetidin
- (3R,4S)-tert-Butyl 2-oxo-4-phenyl-3-(triethylsilyl
- (3R,5S,6E)-7-[2-Cyclopropyl-4-(4-fluorophenyl)-3-q
- (3R,5S,6E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-(N-
- (3R,5S)-7-[2-Cyclopropyl-4-(4-fluorophenyl)-3-quin
- (3R)-3-[4-[(5-Bromo-2-chlorophenyl)methyl]phenoxy]
- (3R)-3-Amino-1-butanol
- (3R)-3-(tert-Butyldimethylsilyl)oxypentanedioate-1
- (3R)-5-Oxo-3-[(triethylsilyl)oxy]-1-cyclopentene-1
- (3S,4a,8aS)-2-[(2R,3S)-3-Amino-2-hydroxy-4-phenylb
- (3S,4R)-3-[(1R)-1-Hydroxyethyl]-4-[(1R)-1-methyl-3
- (3S,4R)-4-(4-Fluorophenyl)-3-hydroxymethyl-1-methy
- (3S,4R)-4-Acetoxy-3-[(R)-1-(tert-butyldimethylsily
- (3S,4S)-3-((R)-(tert-Butyldimethyl-silyloxy)ethyl)
- (3S,4S)-N,4-Dimethyl-1-(phenylmethyl)-3-piperidina
- (3S,5S)-3-(1-Methylethyl)-2-oxo-5-[(2S,4S)-tetrahy
- (3S)-3-[4-[(2-Chloro-5-iodophenyl)methyl]phenoxy]t
- (3S,5S)-5-[(1R,3S)-1-Bromo-3-[[4-methoxy-3-(3-meth
- (3S)-3-[4-[(5-Bromo-2-chlorophenyl)methyl]phenoxy]
- (3S)-3-Amino-2,3,4,5-Tetrahydro-2-Oxo-1H-1-Benzaze
- (3S)-3-Amino-N-cyclopropyl-2-hydroxyhexanamide hyd
- (3S)-3-(tert-Butoxycarbonyl)amino-1-chloro-4-pheny
- (3S)-Tetrahydro-3-Furanyl 4-Methylbenzenesulfonate
- (3Z)-2,3-Dihydro-3-[[[4-[methyl[2-(4-methyl-1-pipe
- (4-[1,2,4] Triazol-1-yl methyl phenyl) hydrazine
- (4-Amino-2-nitrophenyl)carbamic acid ethyl ester
- (4aR,4bS,6aS,7S,9aS,9bS)-2,3,4,4a,4b,5,6,6a,7,8,9,
- (4aR,7aR)-Octahydro-1H-Pyrrolo[3,4-b]Pyridine
- (4aS,7aS)-Octahydro-6-(Phenylmethyl)-1H-Pyrrolo[3,
- (4-Chloro-2-Ethoxycarbonyl)Benzeneboronic Acid
- (4-Chlorophenoxy)acetyl chloride
- (4-Nitrophenyl)methanesulfonyl chloride
- (4-Nitrophenyl)methyl 3-oxobutanoate
- (4R,6R)-6-[2-[2-(4-fluorophenyl)-5-(1-methylethyl)
- (4R,6R)-tert-Butyl-6-(2-aminoethyl)-2,2-dimethyl-1
- (4R,6R)-tert-Butyl-6-cyanomethyl-2,2-dimethyl-1,3-
- (4R,6S)-6-[(1E)-2-[2-Cyclopropyl-4-(4-fluorophenyl
- (4R,12aS)-3,4,6,8,12,12a-Hexahydro-7-hydroxy-4-met
- (4R,12aS)-3,4,6,8,12,12a-Hexahydro-7-methoxy-4-met
- (4R,12aS)-N-[(2,4-Difluorophenyl)methyl]-3,4,6,8,1
- (4R)-4-[[(1,1-Dimethylethyl)Dimethylsilyl]Oxy]-2-C
- (4R)-4-Hydroxy-N,N-diphenyl-2-pentynamide
- (4R)-5-[1,1'-Biphenyl]-4-yl-4-[[(1,1-dimethylethox
- (4R-Cis)-6-[(Acetyloxy) methyl]-2,2-Dimethyl-1,3-D
- (4R-Cis)-6-Hydroxymethyl -2,2-Dimethyl-1,3-Dioxane
- (4R-Cis)-6-Hydroxymethyl-2,2-dimethyl-1,3-dioxane-
- (4S,4aS,5aR,12aS)-9-Amino-4,7-bis(dimethylamino)-1
- (4S,5R)-3-(tert-Butoxycarbonyl)-2,2-dimethyl-4-phe
- (4S,5R)-5-[3,5-Bis(trifluoromethyl)phenyl]-4-methy
- (4S,6S)-4H-Thieno[2,3-b]-thiopyran-4-ol-5,6-dihydr
- (4S,6S)-5,6-Dihydro-4-hydroxy-6-methylthieno[2,3-b
- (4S)-3-[5-(4-Fluorophenyl)-1,5-dioxopenyl]-4-pheny
- (4S)-3-[(2S,4S)-4-Azido-2-(1-methylethyl)-1-oxo-4-
- (4S)-3-[(5S)-5-(4-Fluorophenyl)-5-hydroxypentanoyl
- (4S)-4-Acetamide-5,6-Dihydro-6-Methyl-2-Sulfonamid
- (4S)-(-)-4-Isopropyl-2-oxazolidinone
- (4S-trans) 4H-thieno[2.3-b] thiopyran-4-ol,5,6-dih
- (5a,11b,17b)-11-[4-(Dimethylamino)phenyl]-5,17-dih
- (5alpha,10alpha)-5,10-Epoxy-17-hydroxy-19-norpregn
- (5alpha,11beta)-11-[4-(Dimethylamino)phenyl]-5,17-
- (5-Amino-2-butyl-3-benzofuranyl)[4-[2-(dibutylamin
- (5-Amino-2-butyl-3-benzofuranyl)[4-[3-(dibutylamin
- (5-bromo-2-chlorophenyl)(4-hydroxyphenyl)methanone
- (5-Bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone
- (5-Bromo-2-chlorophenyl)(4-methoxyphenyl)-methanon
- (5R)-3-(3-Fluoro-4-(4-morpholinyl)phenyl)-5-hydrox
- (5R)-3-(4-Bromo-3-fluorophenyl)-5-hydroxymethyloxa
- (5R)-4-(5-Chloro-1,3-benzoxazol-2-yl)-5-methyl-1,4
- (5R)-5-(2,2-Dimethyl-4H-1,3-benzodioxin-6-yl)-1,3-
- (5R-cis)-Toluene-4-sulfonic acid 5-(2,4-difluoroph
- (5S,6E)-7-[4-(4-Fluorophenyl)-6-(1-methylethyl)-2-
- (5S)-2-Oxo-1,5-imidazolidinedicarboxylic acid 5-(1
- (5S)-3-Methyl-2-oxo-1,5-imidazolidinedicarboxylic
- (5S)-N-(Methoxycarbonyl)-L-valyl-5-methyl-L-prolin
- (5Z)-N-(Cyclopropylmethyl)-7-[(1R,2R,3R,5S)-3,5-di
- (6a,11b,16a,17a)-6,9-Difluoro-11,17-dihydroxy-16-m
- (6a,11b,16a,17a)-6,9-Difluoro-11-hydroxy-16-methyl
- (6-Chloro-3-pyridinyl)(4-morpholinyl)methanone
- (6R,7R)-7-Amino-3-[(1Z)-2-(4-methyl-5-thiazolyl)et
- (6R,7R)-7-Amino-3-(chloromethyl)-8-oxo-5-thia-1-az
- (6R,7R)-7-Amino-8-oxo-3-(1-propenyl)-5-thia-1-azab
- (6S)-5,6-Dihydro-6-methyl-4H-thieno[2,3-b]thiopyra
- (6S)-5-Azaspiro[2.4]heptane-5,6-dicarboxylic acid
- (6S)-6-[5-(7-Bromo-9,9-difluoro-9H-fluoren-2-yl)-1
- (6S)-6-[5-[7-[2-(1R,3S,4S)-2-Azabicyclo[2.2.1]hept
- (7a,17b)-7-(9-Bromononyl)-estra-1,3,5(10)-triene-3
- (7a,17b)-7-(9-Bromononyl)estra-1,3,5(10)-triene-3,
- (7a,17b)-7-[9-[(4,4,5,5,5-Pentafluoropentyl)thio]n
- (7alpha,17beta)-17-(Acetyloxy)-7-(9-bromononyl)est
- (7S,9S)9-Acetyl-7,8,9,10-Tetrahydro-6,7,9,11-Tetra
- (8a,9R)-Cinchonan-9-ol mono[[(S)-[(1R)-2-methyl-1-
- (9-[1,1'-Biphenyl]-4-yl-9H-carbazol-3-yl)boronic a
- (9,9-Diphenyl-9H-fluoren-2-yl)boronic acid
- (9b,11b,16a)-9,11-Epoxy-17,21-dihydroxy-16-methyl-
- (9b,11b,16a)-9,11-Epoxy-21-hydroxy-16-methylpregna
- (9-Phenyl-9H-carbazol-3-yl)boronic acid
- 3-[2-(Pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran
- (alphaR)-alpha-(2,4-Difluorophenyl)-alpha-[(1R)-1-
- (alphaR)-alpha-[[[2-(4-Aminophenyl)ethyl]amino]met
- (alphaR)-alpha-[[[2-(4-Nitrophenyl)ethyl]amino]met
- (alphaR)-alpha-Hydroxy-N-[2-(4-nitrophenyl)ethyl]b
- (alphaR)-alpha-[(Methoxycarbonyl)amino]benzeneacet
- (alphaR,gammaS)-gamma-[(3-Carboxy-1-oxopropyl)amin
- (alphaS)-3-Ethoxy-4-methoxy-alpha-[(methylsulfonyl
- (alphaS)-4-Amino-alpha-(3-Amino-3-Oxopropyl)-1,3-D
- (alphaS)-alpha-[[(1R)-2-Hydroxy-1-phenylethyl]amin
- (alphaS)-alpha-Amino-2-chloro-benzeneacetic acid m
- (alphaS)-alpha-Amino-3-ThiopheneAcetic Acid
- (alphaS)-alpha-Aminobenzenebutanoyl-L-leucyl-L-phe
- (alphaS)-alpha-Amino-N-[(1R)-1-[(3aS,4S,6S,7aR)-he
- (alphaS)-alpha-[[[Methyl[[2-(1-methylethyl)-4-thia
- (alphaS,betaR)-beta-(2,5-Difluorophenyl)-beta-hydr
- (alphaS,betaS)-beta-Amino-alpha-[[1-[[4-(2-pyridin
- (aR,3aS,4S,6S,7aR)-Hexahydro-3a,8,8-trimethyl-alph
- (Benzothiazol-2-yl)-(Z)-2-trityloxyimino-2-(2-amin
- (betaR)-beta-Cyclopentyl-4-(4,4,5,5-tetramethyl-1,
- (betaR)-beta-Cyclopentyl-4-[7-[[2-(trimethylsilyl)
- (betaR,gammaR)-gamma-Ethyl-3-methoxy-N,N,beta-trim
- (betaS)-beta-Amino-3-ethoxy-4-methoxybenzeneethano
- (Bromomethyl)cyclopropane
- (E)-1,4-Dibromobut-2-ene
- (E)-3-(3-(N-phenylsulfamoyl)phenyl)acrylic acid
- (E)-3-[2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolin
- (E)-3-[3'-(4-Fluorophenyl)-1'-(1-methylethyl)-1H-i
- (E)-3-[3'-(4''-Fluorophenyl)-1'-(1''-methylethyl)-
- (E)-4-(4-Nitrophenyl)But-3-En-2-One
- (E)-6-Iodo-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahyd
- (E)-6-Nitro-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahy
- (E)-7-[2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolin
- (E)-()-3-methyl-1-(2,6,6-trimethylcyclohex-1-en-1
- (E)-methyl6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,
- (E)-N-(2-Chloro-6-methylphenyl)-3-ethoxyacrylamide
- (E)-triMethyl((1-iodo-4-Methyloct-1-en-4-yl)oxy)si
- (N,N-dimethyl-3-(naphthalen-1-yloxy)-3-(thiophen-2
- (Phenylazo)malonitrile
- (R) -3- amino-1- ( 3- ( trifluoromethyl ) -5, 6-di
- (R)-(-)-1,3-Butanediol
- (R)-(-)-1-Aminoindane hydrochloride
- (R)-(-)-2,2-Dimethyl-1,3-dioxolane-4-methanol
- (R)-(-)-2-Amino-1-butanol
- (R)-(-)-2-Amino-3-methyl-1-butanol
- (R)-(-)-2-Chloromandelic acid
- (R)-(-)-2-Hydroxymethylpyrrolidine
- (R)-(-)-3-Carbamoymethyl-5-methylhexanoic acid
- (R)-(-)-3-Chloro-1,2-propanediol
- (R)-(-)-3-Quinuclidinol
- (R)-(-)-4-Phenyl-2-oxazolidinone
- (R)-(-)-alpha-(Trifluoromethyl)benzylamine
- R)-(-)-alpha-Methoxyphenylacetic acid
- (R)-(-)-Epichlorohydrin
- (R)-(-)-gamma-Toluenesulfonylmethyl-gamma-Butyrolactone
- (R)-(-)-O-Formylmandeloyl chloride
- (R)-(+)-1-(1-Naphthyl)ethylamine
- (R)-(+)-1,1'-Bi-2-naphthol
- (R)-(+)-1-Benzyl-3-pyrrolidinol
- (R)-(+)-1-Boc-3-aminopyrrolidine
- (R)-(+)-1-Phenylethylamine
- (R)-(+)-2-(4-Hydroxyphenoxy)propionic acid
- (R)-(+)-2-(Diphenylhydroxymethyl)pyrrolidine
- (R)-(+)-2,2-Dimethyl-1,3-dioxolane-4-carboxaldehyde
- (R)-(+)-4-Isopropyl-2-oxazolidinone
- (R)-(+)-9-(2-Hydroxypropyl)adenine
- (R)-(+)-Glycidol
- (R)-(+)-N-Benzyl-1-phenylethylamine
- (R)-(+)-Propylene carbonate
- (R)-[3-(3-Fluoro-4-morpholinophenyl)-2-oxo-5-oxazolidinyl]methyl methanesulfonate
- (R)-1-(2,6-Dichloro-3-fluorophenyl)ethanol
- (R)-1,4-Dioxaspiro[4,5]decane-2-carboxaldehyde
- (R)-1-[(S)-2-(Diphenylphosphino)ferrocenyl]ethyldi-tert-butylphosphine
- (R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethanol
- (R)-1-Boc-3-Aminopiperidine
- (R)-1-Boc-3-Hydroxypiperidine
- (R)-1-N-Boc-2-methylpiperazine
- (R)-2-[(5-Bromo-1H-indol-3-yl)carbonyl]-1-pyrrolidinecarboxylic acid benzyl ester
- (R)-2-[3-(Diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)phenol fumaric acid salt
- (R)-2-Amino-N-benzyl-3-methoxypropanamide
- (R)-2-Hydroxybutyric acid
- (R)-2-Methyl-pyrrolidine
- (R)-2-Methylpyrrolidine hydrochloride
- (R)-3-(3-Methylbutanoyl)-4-benzyloxazolidin-2-one
- (R)-3-(4-Phenoxyphenyl)-1-(piperidin-3-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine
- (R)-3-(Boc-Amino)piperidine
- (R)-3-amino-1,2,3,4-tetrahydrocarbazole
- (R)-3-Amino-2-azepanone
- (R)-3-Amino-3-benzo[1,3]dioxol-5-ylpropionic acid
- (R)-3-Amino-4-(2,4,5-trifluorophenyl)butyric acid
- (R)-3-Aminopiperidine dihydrochloride
- (R)-3-Aminoquinuclidine dihydrochloride
- (R)-3-Hydroxypiperidine hydrocloride
- (R)-3-Quinuclidinol hydrochloride
- (R)-4-[4-[(3-Chloro-2-hydroxypropyl)amino]phenyl]morpholin-3-one
- (R)-4-Benzyl-2-oxazolidinone
- (R)-5-(Azidomethyl)-3-[3-fluoro-4-(4-morpholinyl)phenyl]-2-oxazolidinone
- (R)-5-Bromo-3-((1-methylpyrrolidin-2-yl)methyl)-1H-indole
- (R)-5-Hydroxymethyldihydrofuran-2-one
- (R)-6-(4-Aminophenyl)-4,5-dihydro-5-methyl-3(2H)-pyridazinone
- (R)-6-Fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylicacid
- (R)-alpha-Methoxy-alpha-(trifluoromethyl)phenylacetyl chloride
- (R)-alpha-Methyl-4-nitrobenzylamine
- (R)-Glycidyl butyrate
- (R)-Homo-beta-alanine hydrochloride
- (R)-Mandelic acid
- (R)-N-(2,3-Epoxypropan-1-yl)phthalimide
- (R)-N-Benzyl-2-(benzyloxycarbonylamino)-3-hydroxypropionamide
- (R)-N-Boc-(3-Pyridyl)alanine
- (R)-N-Boc-(4-Pyridyl)alanine
- (R)-O-Acetylmandelic acid chloride
- (R)-Styrene oxide
- (R)-Tetrahydropapaverine N-acetyl-L-leucinate
- (R,R)-(-)-N,N'-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine
- (R,R)-1,2-Bis(methanesulfonyloxymethyl)cyclohexane
- (R,R)-1,2-Cyclohexanedicarboxylic anhydride
- (R,R)-7-AMINO-3-(1-METHYLPYRROLIDINIO)METHYL-3-CEPHEM-4-CARBOXYLATE HCL
- (S)-((2-Guanidino-4-thiazolyl)methylisothiourea dihydrochloride
- (S)-(-)-1-(1-Naphthyl)ethylamine
- (S)-(-)-1-(4-Methoxyphenyl)ethylamine
- (S)-(-)-1,2,3,4-Tetrahydro-naphthoic acid
- (S)-(-)-1,2-Diaminopropane dihydrochloride
- (S)-(-)-1-Boc-2-pyrrolidinemethanol
- (S)-(-)-1-Boc-3-aminopyrrolidine
- (S)-(-)-2-(Diphenylhydroxymethyl)pyrrolidine
- (S)-(-)-2-Chloropropionic acid
- (S)-(-)-3-(Benzoylthio)-2-methylpropanoic acid
- (S)-(-)-3-Amino-3-phenylpropionic acid hydrochloride
- (S)-(-)-4-Amino-2-hydroxybutyric acid
- (S)-(-)-Indoline-2-carboxylic acid
- (S)-(-)-N,N-Dimethyl-3-hydroxy-3-(2-thienyl)propanamine
- (S)-(-)-N-Benzyl-1-phenylethylamine
- (S)-(+)-2,2-Dimethyl-1,3-dioxolane-4-methanol
- (S)-(+)-2,2-Dimethylcyclopropanecarboxamide
- (S)-(+)-2-Amino-3-methyl-1-butanol
- (S)-(+)-2-Chlorophenylglycine methyl ester
- (S)-(+)-2-Methylpiperazine
- (S)-(+)-2-Phenylglycinol
- (S)-(+)-2-Phenylpropionic acid
- (S)-(+)-3-Hydroxytetrahydrofuran
- (S)-(+)-3-Quinuclidinol
- (S)-(+)-Dihydro-5-(p-Tolylsulfonyloxymethyl)-2(3H)-Furanone
- (S)-(+)-Epichlorohydrin
- (S)-(+)-N-(2,3-Epoxypropyl)phthalimide
- (S)-(+)-O-Acetyl-L-mandelic acid
- (S)-[1-(3-Mercapto-1-pyrrolidinyl)ethylidene]carbamic acid (4-nitrophenyl)methyl ester
- (S)-1-(2,4-Dichlorophenyl)-1,2-ethanediol
- (S)-1-(2,4-Dichlorophenyl)-1,2-ethanediol dimethanesulfonate
- (S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol
- (S)-1-(2-Amino-5-chlorophenyl)-1-(trifluoromethyl)-3-cyclopropyl-2-propyn-1-ol
- (S)-1-(2-Chloroacetyl)pyrrolidine-2-carboxamide
- (S)-1-(3-Ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethylamine N-acetyl-L-leucine salt
- (S)-1-(3-Methoxyphenyl)ethylamine
- (S)-1-(4-Chlorophenyl)ethylamine
- (S)-1-(4-Nitrophenyl)ethylamine hydrochloride
- (S)-1,2,3,4-Tetrahydro-5-methoxy-N-propyl-2-naphthalenamine hydrochloride
- (S)-1-[(Acetylamino)methyl]-2-chloroethyl acetate
- (S)-10-Hydroxycamptothecin
- (S)-1-Amino-3-chloro-2-propanol hydrochloride
- (S)-1-Benzyl-3-pyrrolidinol
- (S)-1-Boc-2-cyanopyrrolidine
- (S)-1-Boc-3-hydroxypiperidine
- (S)-1-N-Boc-2-methylpiperazine
- (S)-2-(((9H-Fluoren-9-yl)methoxy)carbonylamino)-6-aminohexanoic acid
- (S)-2-(1,6,7,8-Tetrahydro-2H-indeno[5,4-b]furan-8-yl)ethylamine
- (S)-2-(3-((2-Isopropylthiazol-4-yl)methyl)-3-methylureido)-3-methylbutanoic acid
- (S)-2-(4-Fluorophenyl)-3-Methylbutyric Acid
- (S)-2-(Aminomethyl)-1-ethylpyrrolidine
- (S)-2-(Benzyloxycarbonylamino)-3-butenoic acid methyl ester
- (S)-2,2-Diphenyl-2-(pyrrolidin-3-yl)acetonitrile hydrobromide
- (S)-2,4-Dichloro-alpha-(chloromethyl)-benzenemethanol
- (S)-2-[(4-Chlorophenyl)(4-piperidinyloxy)methyl]pyridine
- (S)-2-Amino-2'-hydroxy-1,1'-binaphthyl
- (S)-2-Amino-5-methoxytetrahydronaphthalene hydrochloride
- (S)-2-Amino-5-methoxytetralin (S)-mandelate
- (S)-2-Amino-5-Methoxytetralin Hydrochloride
- (S)-2-Benzothiazolyl (Z)-2-(2-aminothiazole-4-yl)-2-methoxycarbonylmethoxyiminothioacetate
- (S)-2-Benzothiazolyl (Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-methoxyiminothioacetate
- (S)-2-Benzyl-N,N-dimethylaziridine-1-sulfonamide
- (S)-2-Benzylsuccinic acid
- (S)-2-chloro-1-(2,4-dichlorophenyl)ethyl methanesulfonate
- (S)-2-Chloromandelic acid
- (S)-2-Hydroxy-3-methoxy-3,3-diphenylpropionic acid
- (S)-2-Hydroxybutyric acid
- (S)-3-(1-Cyano-1,1-diphenylmethyl)-1-tosylpyrrolidine
- (S)-3-(2-Thienylthio)butanoic acid
- (S)-3-(4-Methoxy-3-sulfonamidophenyl)-2-propylamine Hydrochloride
- (S)-3-(Benzyloxycarbonyl)-4-isopropyl-2,5-oxazolidinedione
- (S)-3-(Boc-amino)-4-phenylbutyric acid
- (S)-3,4-Dihydro-6-chloro-4-hydroxy-2H-thieno[3,2-e]-1,2-thiazine-1,1-dioxide
- (S)-3-amino-1,2,3,4-terahydrocarbazole
- (S)-3-Amino-1-N-Boc-piperidine
- (S)-3-Amino-3-phenylpropan-1-ol
- (S)-3-Amino-3-phenylpropanoic acid ethyl ester hydrochloride
- (S)-3-Aminoquinuclidine dihydrochloride
- (S)-3-Dimethylamino-1-(3-methoxyphenyl)-2-methyl-1-propanone
- (S)-3-Hydroxypyrrolidine hydrochloride
- (S)-3-Methyl-1-(2-piperidinophenyl)butylamine N-acetylglutamate salt
- (S)-3-Phenoxybenzaldehyde cyanohydrin
- (S)-3-tert-Butylamino-1,2-propanediol
- (S)-4-(4-(5-(Aminomethyl)-2-oxooxazolidin-3-yl)phenyl)morpholin-3-one
- (S)-4-(4-Aminobenzyl)-2(1H)-oxazolidinone
- (S)-4-(4'-Nitrobenzyl)-1,3-oxazolidine-2-one
- (S)-4,5,6,7-Tetrahydro-2,6-benzothiazolediamine
- (S)-4-Benzyl-2-oxazolidinone
- (S)-4-Nitro-alpha-methylbenzylamine
- (S)-5-(Aminomethyl)-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one
- (S)-5-(tert-Butoxycarbonyl)-5-azaspiro[2.4]heptane-6-carboxylic acid
- (S)-5,6,7,8-Tetrahydro-6-(propylamino)-1-naphthalenol hydrobromide
- (S)-5-[(Biphenyl-4-yl)methyl]-1-(2,2-dimethylpropionyl)pyrrolidin-2-one
- (S)-5-[(Biphenyl-4-yl)methyl]pyrrolidin-2-one
- (S)-5-Hydroxymethyldihydrofuran-2-one
- (S)-5-Methoxy-N-propyl-N-(2-(thiophen-2-yl)ethyl)-1,2,3,4-tetrahydronaphthalen-2-amine
- (S)-6-Fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid
- (S)-6-Oxo-2-piperidinecarboxylic acid
- (S)-8-Chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine
- (S)-alpha,alpha-Diphenyl-3-pyrrolidineacetamide
- (S)-alpha-Ethyl-2-oxo-1-pyrrolidineacetic acid (R)-alpha-methylbenzenemethanamine salt
- (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]phthalimide
- (S)-N-Boc-2-pyrrolidone-5-carboxylic acid tert-butyl ester
- (S)-N-Carbobenzyloxy-4-amino-2-hydroxybutyric acid
- (S)-Pyrrolidine-2-carbonitrile hydrochloride
- (S)-tert-Butyl 3-oxo-1-phenylpropylcarbamate
- (S,S)-2,8-Diazabicyclo[4.3.0]nonane
- (S,S)-2-Azabicyclo[3,3,0]-octane-3-carboxylic acid benzylester hydrochloride
- (S,S,S)-2-Azabicyclo[3,3,0]-octane-carboxylic acid benzylester hydrochloride
- (S,Z)-5-Amino-2-(dibenzylamino)-1,6-diphenylhex-4-en-3-one
- (SP-4-3)-Dimethyl(1,1,1-Trifluoro-2,4-Pentanedionato)-Gold
- (trans,trans)-4-broMo-4'-pentyl-1,1'-Bicyclohexane
- (trans,trans)-4'-Propyl-[1,1'-bicyclohexyl]-4-methanol
- (Z)-(2,4-Difluorophenyl)-4-piperidinylmethanone oxime
- (Z)-2-(2-Aminothiazol-4-yl)-2-(1-carboxy-1-methyl)ethoxyiminoacetic acid
- (Z)-2-(2-Aminothiazol-4-yl)-2-(tert-butoxycarbonylmethoxyimino)acetic acid
- (Z)-2-(2-Aminothiazol-4-yl)-2-carboxymethoxyiminoacetic acid
- (Z)-2-(2-tert-Butoxycarbonylaminothiazol-4-yl)-2-pentenoic acid
- (Z)-2-(2-Tritylaminothiazol-4-yl)-2-(2-tert-butoxycarbonylprop-2-oxyimino)acetic acid
- (Z)-2-Amino-alpha-[1-(tert-butoxycarbonyl)]-1-methylethoxyimino-4-thiazolacetic acid
- (Z)-2-Amino-alpha-propylidene-4-thiazoleacetic acid methyl ester hydrochloride
- (Z)-2-Methoxyimino-2-(furyl-2-yl) acetic acid ammonium salt
- (Z)-4-Chloro-2-[(2-methoxy-2-oxoethoxy) imino]-3-oxobutanoic acid
- (Z)-5-Amino-alpha-(ethoxyimino)-1,2,4-thiadiazole-3-acetic acid
- [(1R)-2-(Biphenyl-4-yl)-1-formylethyl]carbamic acid tert-butyl ester
- [(1S)-1-[[(2-Fluoro-6-nitrobenzoyl)phenylamino]carbonyl]propyl]carbamic acid 1,1-dimethylethyl ester
- [(1S)-3-Methyl-1-[[(2R)-2-methyloxiranyl]carbonyl]butyl]carbamic acid 1,1-dimethylethyl ester
- [(1S,2R)-2-[3,5-Bis(trifluoromethyl)phenyl]-2-hydroxy-1-methylethyl]carbamic acid phenylmethyl ester
- [(1S,3S,4S)-4-Amino-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]carbamic acid 1,1-dimethylethyl ester
- [(2,6-Dimethylphenyl)Aminocarbonylmethyl]Chloride
- [(2-Methyl-1-propionylpropoxy)(4-phenylbutyl)phosphinoyl]acetic acid
- [(2S,5R)-5-(5-Methyl-2,4-Dioxopyrimidin-1-Yl)-2,5-Dihydrofuran-2-Yl]Methyl Benzoate
- [(3S,4R)-1-Ethyl-4-(4-Fluorophenyl)-3-Piperidinyl]Methanol
- [(7S)-5-(Phenylmethyl)-5-azaspiro[2.4]hept-7-yl]carbamic acid tert-butyl ester
- [[(Carboxymethyl)amino]methylene]propanedioic acid 1,3-diethyl ester
- [[2-(6-Amino-9H-purin-9-yl)ethoxy]methyl]phosphonic acid diethyl ester
- [2-Cyclopropyl-4-(4-fluorophenyl)-quinolin-3-ylmethyl]-triphenyl-phosphonium bromido
- [[4-(2-Methoxyethyl)phenoxy]methyl]oxirane
- [[4-[[2-(1-Methylethoxy)ethoxy]methyl]phenoxy]methyl]oxirane
- [[4-[2-(Cyclopropylmethoxy)ethyl]phenoxy]methyl]oxirane
- [[5-(3-Fluorophenyl)-2-pyridinyl]methyl]phosphonic acid diethyl ester
- [1,1'-Biphenyl]-3-carboxylic acid, 5'-chloro-2'-hydroxy-
- LT-BPK(BiMaprostLatanprost)
- [2-(2-Phenoxyethoxy)Ethyl]Diethylamine
- [2-(4-Aminophenyl)ethyl]carbamic acid tert-butyl ester
- [2,8-Bis(trifluoromethyl)-4-quinolinyl]-2-pyridinylmethanone
- [2-[(5-Chloro-benzooxazol-2-yl)(3-oxobutyl)amino]ethyl]carbamic acid tert-butyl ester
- 11beta,21-dihydroxypregna-1,4,16-triene-3,20-dione 21-acetate
- [2-[2-(triphenylmethyl)-2H-tetrazol-5-yl]phenyl]boronic acid
- 5-Methylcyclocytidine Hydrochlorine
- [2S,3S,5S]-2-Amino-3-hydroxy-5-tert-butyloxycarbonylamino-1,6-diphenylhexane succinate salt
- [3-(Dimethylamino)propyl]triphenylphosphonium bromide hydrobromide
- [4-(2-Phenyl-1H-benzimidazol-1-yl)phenyl]boronic acid
- [4-[4-(4-Hydroxyphenyl)-1-piperazinyl]phenyl]carbamic acid phenyl ester
- [5-Bromo-3-[(1R)-(2,6-dichloro-3-fluorophenyl)ethoxy]pyridin-2-yl]amine
- [6-Methyl-2-(4-methylphenyl)imidazo[1,2-a]pyridin-3-yl]acetonitrile
- [S-(R*,R*)]- 2,2,2-Trifluoro-N-(1-oxiranyl-2-phenylethyl)acetamide
- 1-({[(3S)-Tetrahydro-3-Furanyloxy]Carbonyl}Oxy)-2,5-Pyrrolidinedione
- 1-(1,4-Benzodioxane-2-carbonyl)piperazine
- 1-(2-(4-(Chloromethyl)phenoxy)ethyl)azepane hydrochloride
- 1-(2,2-Difluorobenzodioxol-5-yl)cyclopropanecarboxylic acid
- 1-(2,2-Dimethoxyethyl)-1,4-dihydro-3-methoxy-4-oxo-2,5-pyridinedicarboxylic acid 2-methyl ester
- 1-(2,3-Dichlorophenyl)piperazine
- 1-(2,3-Dichlorophenyl)piperazine Hydrochloride
- 1-(2,3-Dihydro-1,4-benzodioxin-2-yl)ethan-1-one
- 1-(2,3-Dihydro-1,4-benzodioxin-2-ylcarbonyl)piperazine hydrochloride
- 1-(2,5-Difluorophenyl)-2-(1H-1,2,4-triazol-1-yl)ethanone
- 1-(2,6-Dichlorophenyl)-2-indolinone
- 1-(2,6-Dichlorophenyl)indolin-2-one
- 1-(2-Amino-3-bromo-4-methoxyphenyl)ethanone
- 1-(2-Amino-3-Chlorophenyl)-Ethanone
- 1-(2-Amino-3-Methoxy-Phenyl)Ethanone
- 1-(2-Amino-3-Methylphenyl)-Ethanone
- 1-(2-Amino-4-bromo-5-fluorophenyl)ethanone
- 1-(2-Amino-4-chloro-5-fluorophenyl)ethanone
- 1-(2-AMino-4-nitro-phenyl)-ethanone
- 1-(2-Amino-5-bromo-4-chlorophenyl)ethanone
- 1-(2-Aminoethyl)pyrrolidine
- 1-(2-Benzyloxy-Ethyl)-Piperazine
- 1-(2-Bromoethoxy)-4-nitrobenzene
- 1-(2-Chloro-4-(4-chlorophenyl)butyl)-1H-imidazole
- 1-(2-Chloroethyl)-1H-1,2,4-triazole
- 1-(2-Chloroethyl)-2-Methoxybenzene
- 1-(2-Chloroethyl)-4-methylpiperazine dihydrochloride
- 1-(2-Chloroethyl)azepane hydrochloride
- 1-(2-Fluoro-4-nitrophenyl)piperazine
- 1-(2-Fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carbonitrile
- 1-(2-Fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxaMide
- 1-(2-Fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboximidamide hydrochloride
- 1(2H)-Phthalazinone
- 1-(2-Hydroxyethyl)-4-methylpiperazine
- 1-(2-Iodoethyl)-4-octylbenzene
- 1-(2-Methoxyethyl)piperazine
- 1-(2-Methoxyphenyl)piperazine Hydrobromide
- 1-(2-Methoxyphenyl)piperazine Hydrochloride
- 1-(2-Pyrimidyl)piperazine Hydrochloride
- 1-(3-Carboxypyrid-2-yl)-2-phenyl-4-methyl-piperazine
- 1-(3-Chlorophenyl)-4-(3-chloropropyl)piperazine
- 1-(3-Chlorophenyl)-4-(3-Chloropropyl)Piperazine HCL
- 1-(3-Chlorophenyl)-4-(3-chloropropyl)piperazine hydrochloride
- 1-(3-Chlorophenyl)piperazine Dihydrochloride
- 1-(3-Chlorophenyl)piperazine Hydrochloride
- 1-(3-Chlorophenyl)piperazinium Chloride
- 1-(3-Chloropropyl)-1,3-dihydro-2H-benzimidazol-2-one
- 1-(3-Chloropropyl)-4-methylpiperazine dihydrochloride
- 1-(3-Chloropropyl)piperidine Monohydrochloride
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
- 1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethanol
- 1-(3-Ethyl-4-hydroxymethyl-phenyl)ethanone
- 1-(3-Hydroxypropyl)-4-Methylpiperazine
- 1-(3-Methyl-1-phenyl-5-pyrazolyl)piperazine
- 1-(3-Pyridyl)-3-(dimethylamino)-2-propen-1-one
- 1-(4-(1-PHENETHYL-1H-IMIDAZOLE-2-CARBONYL)PIPERIDIN-1-YL)ETHANONE
- 1-(4,5-Dihydro-2-thiazolyl)-3-azetidinethiol hydrochloride
- 1-(4-Amino-2-methylbenzoyl)-7-chloro-1,2,3,4-tetrahydro-5H-1-benzazepin-5-one
- 1-(4-Amino-3-Bromo-Phenyl)-Ethanone
- 1-(4-Aminophenyl)-2,3-Dihydro-1,3,3-Trimethyl-1H-Inden-5-Amine
- 1-(4-Aminophenyl)-2-Bromoethanone
- 1-(4-Aminophenyl)-4-(4-methoxyphenyl)piperazine
- 1-(4-Aminophenyl)-5,6-dihydro-3-(4-morpholinyl)-2(1H)-pyridinone
- 1-(4-Bromo-3-fluorophenyl)ethanone
- 1-(4-Bromophenyl)-2-phenylbenzimidazole
- 1-(4-BROMO-PHENYL)-PIPERIDIN-2-ONE
- 1-(4-Chlorobenzhydryl)piperazine
- 1-(4-Chlorophenyl)-1-cyclobutanecarbonitrile
- 1-(4-Chlorophenyl)-2-cyclopropylpropan-1-one
- 1-(4-Chlorophenyl)-2H-pyrazolin-3-one
- 1-(4-Chlorophenyl)-4,4-dimethyl-3-pentanone
- 1-(4-Fluorobenzyl)-2-chlorobenzimidazole
- 1-(4-Fluorophenethyl)-4-piperidone
- 1-(4-Fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitile
- 1-(4-Fluorophenylcarbamoyl)cyclopropanecarboxylic acid
- 1-(4-Hydrazinophenyl)methyl-1,2,4-triazole
- 1-(4-Hydroxybutyl)-4-Methylpiperazine
- 1-(4-Iodophenyl)-2-piperidinone
- 1-(4-Iodophenyl)-3-morpholino-5,6-dihydropyridin-2(1H)-one
- 1-(4-Methoxyphenyl)-2-benzylaminopropane
- 1-(4-Methoxyphenyl)-2-phenylethanone
- 1-(4-Methoxyphenyl)-4-(4-nitrophenyl)piperazine
- 1-(4-Methoxyphenyl)piperazine Dihydrochloride
- 1-(4-Nitrophenyl)methyl-1,2,4-triazole
- 1-(4-Pyridyl)acetone
- 1-(5-Amino-2-fluorophenyl)ethanone
- 1-(5-Bromo-2-fluorophenyl)ethanone
- 1-(5-Bromo-2-methoxy-phenyl)adamantane
- 1-(6-Methylpyridin-3-yl)-2-[4-(methylsulfonyl)phenyl]ethanone
- 1-(7-Bromo-9,9-difluoro-9H-fluoren-2-yl)-2-chloroethanone
- 1-(Benzyloxy)-2-(Chloromethyl)Benzene
- 1-(Bromoacetyl)pyrene
- 1-(Cbz-amino)cyclopropanecarboxylic Acid
- 1-(Cyanomethyl)imidazole
- 1-(cyanomethyl)imidazole Hydrochloride
- 1-(Cyclopropylcarbonyl)piperazine
- 1-(Dimethylamino)-2-Methyl-3-Pentanone
- 1-(Dimethylamino)-3-[2-[2-(3-methoxyphenyl)ethyl]phenoxy]-2-propanol hydrochloride
- 1-(Diphenylmethyl)-3-hydroxyazetidine
- 1-(Diphenylmethyl)piperazine
- 1-(Ethoxycarbonyl)piperidine-4-carboxylic acid
- 1-(Hydroxycyclohexyl)-(4-methoxyphenyl)acetonitrile
- 1-(Hydroxymethyl)cyclopropaneacetonitrile
- 1-(Piperidin-4-yl)-1,3-dihydrobenzoimidazol-2-one
- 1-(tert-Butyl) 2-methyl (2R,4S)-4-hydroxypiperidine-1,2-dicarboxylate
- 1-(Toluene-4-sulfonyl)-2,5-dihydro-1H-pyrrole
- 1-(Trifluoromethyl)-1,2-benziodoxol-3(1H)-one
- 1,1'-(4-Chlorobutylidene)Bis(4-Fluorobenzene)
- 1,1'-(Azodicarbonyl)-dipiperidine
- 1,1,1-Trifluoro-2,3-epoxypropane
- 1,1,1-Trimethoxy-2-methylpropane
- 1,1,1-Trimethyl-N-(trimethylsilyl)silanamine
- 1,1,2,2-Tetrachloroethane
- 1,1,2-Trichloroethane
- 1,1,2-Trimethoxyethane
- 1,1,2-Trimethyl-1H-benz[e]indole
- 1,1,3,3-Tetramethoxypropane
- 1,10-Phenanthroline Hydrate
- 1,10-Phenanthroline-5,6-dione
- 1,1-Bis(4-diethylaminophenyl)-4,4-diphenyl-1,3-butadiene
- 1,1-Bis(hydroxymethyl)cyclopropane
- 1,1'-Carbonyldiimidazole
- 1,1-Cyclobutanedicarboxylic Acid
- 1,1-Cyclohexanediacetic Acid
- 1,1-Cyclohexanediacetic acid mono amide
- 1,1-Dimethoxy-2-(2-methoxyethoxy)ethane
- 1,1-Dimethyl-1,2-ethanediamine
- 1,1-Dimethylpropargylamine
- 1,1-Diphenylethanol
- 1,1'-Thiocarbonyldiimidazole
- 1,2,3,4a,9,9a-Hexahydro-9-methyl-4H-carbazole-4-one
- 1,2,3,4-Tetrahydro-1-naphthoic Acid
- 1-(4-chloro-3-(trifluoromethyl)phenyl)-3-hydroxyurea
- CAUSTIC SODA LYE
- CAUSTIC SODA FLAKE
- POTASSIUM PERMANGANATE
- BORIC ACID
- HP- Phosphinidene
- KOH - Potassium hydroxide
- Chloroform .
- MDC - Dichloromethane
- POCl3 .
- TC -Technetium
- Industrial Chemicals
- Speciality chemicals
- Chemicals
- Nutraceuticals & Food chemicals
- Fine chemicals
- Citric Acid
- Dyes chemicals
- Octanol
- Herbal extracts & chemicals
- Para Octyl Phenol
- Potassium Phthalimide
- Pyrrolidine
- Pyridinium P Toluenesulfonate
- Sodium Thiosulphate
- Potassium Hydroxide Pellets
- 2,4-dichlorophenol
- 12 hydroxy stearic acid
- Barium Sulfate
- Acetic Acid Chemical
- Acetone
- Acrylic acid
- Aluminum Chloride
- Ammonium Molybdate
- Basic chromium sulfate
- Boric acid
- Butyl carbitol
- Calcium Chloride Dihydrate
- Phosphorus Oxychloride
- Phosphorus Pentachloride
- Phosphorus Pentasulfide
- phosphorus pentoxide
- Phosphorous Tri Chloride
- Potassium Carbonate
- Potassium Carbonate Granules
- Potassium Iodide
- Potassium Permanganate
- Propylene Glycol
- Pyridine
- resolving agents
- Soap noodles
- Sodium Bisulfite
- Sodium Gluconate
- Sodium Hydroxide Pellets
- Sodium Iodide
- Sodium Metabisulphite
- Sodium Methoxide Solution
- Sodium methoxide powder solution
- Sodium Molybdate
- Sodium Stannate
- Sodium Sulphate
- Sodium Tungstate
- stannic chloride anhydrous
- Stannous Chloride Anhydrous
- Stannous Chloride Dihydrate
- Iodine
- Thionyl chloride
- Butylated hydroxytoluene (bht)
- Iso Butanol
- Calcium Chloride
- Caustic Potash Flakes
- Isophorone
- Chloroform
- Chlorosulphonic Acid
- Cobalt Acetate
- Isopropyl alcohol
- Cobalt chloride
- Bromine Solution
- Cobalt Sulfate
- Copper Sulfate
- Cyclohexane
- liquid cyloride
- cyclohexanone
- Liquid Toluene
- dicyandiamide
- dimethyl formamide
- lithium carbonade
- Dimethyl sulfoxide
- Dimethyl Sulphate
- Lithium Chloride
- Lithium Molybdate
- Lithium Nitrate
- Litium bromine
- Malinonitrile .
- ethyl acetate
- Formaldehyde
- Methylene Chloride
- Methyl Ethyl Ketone
- Methyl Isobutyl Ketcone
- Methyl Salicylate
- Nickel Metal
- Nickel sulpate
- N- methylpiperazine
- Nonylphenol
- normal butanol
- Formic Acid
- octanol
- Hydrogen Peroxide
- Oleum
- Hydroxylamine Hydrochloride
- Ortho chloro penol
- Oxalic Acid
- Oxalyl Cloride
- Tetrahydrofuran
- Paraffin Wax
- Tetrasodium Pyrophosphate
- Phosphoric Acid
- phosphorous oxychloride
- Phosphorus pentachloride
- phosphorus pentasulphide
- Trimethyl Orthoformate
- trimethyl sulfoxonium iodide
- Zinc Chloride Powder
- Zinc Oxide
- Stannous Fluoride
- Stannous Oxalate
- Stannous Oxide
- Stannous Sulphate
- Stearic Acid
- Sulfur Powder
- Sulfuryl Chloride
- Sulphanilic acid
- Sulphur Dioxide
- Sulphur Granules
- Sulphuric Acid
- Sulphuryl Chloride
- Asparaginase
- Barnidipine hydrochloride
- Berberine hydrogen sulphate
- Bexarotene
- Buparvaquone
- Cefozopran Hydrochloride
- Dextropropoxyphene Hydrochloride
- 1,1,1-Trichloro-2-methyl-2-propanol Hemihydrate
- Intermediates
- Pharmaceutical Excipients
- Herbal Extracts
- Nutraceutical Ingredients
- Active Pharmaceutical Ingredient
- Industry Served
- Contact Us
Isepamicin sulfate
Product Details:
- Loss on Drying 4.0%
- Shelf Life 2 years
- Color White to off-white
- Poisonous Non-poisonous as per standard pharmaceutical usage
- Particle Size Suitable for injection/reconstitution (typically micronized)
- Solubility Freely soluble in water, practically insoluble in ethanol and ether
- Heavy Metal (%) 20 ppm
- Click to View more
X
Isepamicin sulfate Price And Quantity
Isepamicin sulfate Product Specifications
- White to off-white powder
- Used for the treatment of bacterial infections
- Freely soluble in water, practically insoluble in ethanol and ether
- Non-poisonous as per standard pharmaceutical usage
- Suitable for injection/reconstitution (typically micronized)
- 71835-48-6
- White to off-white
- 2 years
- 4.0%
- 181-183C (dec.)
- Isepamicin sulfate
- Pharmaceutical Grade
- 276-177-1
- Characteristic
- 29419090
- 621.67 g/mol
- 98.0%
- Available on request
- Isepamicin sulfate
- 6.0-8.0 (1% solution)
- C22H43N5O12S
- Store at 2-8C in a dry, dark place, tightly closed
- Odorless
- Solid
- Antibiotic
- 20 ppm
Product Description
CAS number: 67814-76-0
Pharmacopeia: CP,JP,IN-HOUSE
Certificate: ISO CERTIFICATE,GMP,FACTORY GMP,WHO GMP,USFDA,EUGMP,GMP-SFDA,cGMP,PRODUCT GMP,Cofepris Mexico,Halal,Kosher,Korea FDA
Properties :
| Synonym | (2S)-3-amino-N-[(1R,2S,3S,4R,5S)-5-amino-4-[(2R,3R,4S,5S,6R)-6-(aminomethyl)-3,4,5-trihydroxy-oxan-2-yl]oxy-2-[(2R,3R,4R,5S)-3,5-dihydroxy-5-methyl-4-methylamino-oxan-2-yl]oxy-3-hydroxy-cyclohexyl]-2-hydroxy-propanamide sulfate |
| Molecular Formula | C22H43N5O12.H2SO4 |
| Molecular Weight | 667.68 |
| Specifications | AVAILABLE ON REQUEST |
| Packing | EXPORT WORTHY PACKING |
| Availability | FOR R&D PURPOSE ONLY |
Pharmaceutical Excellence
Isepamicin sulfate is manufactured under robust quality control measures, adhering strictly to USP, EP, and JP standards. Its exceptional purity and microbe-free guarantee make it a preferred choice in critical healthcare environments for injectable antibiotic therapy.
Optimized for Injection
The carefully micronized particle size and freely soluble nature of Isepamicin sulfate ensure reliable reconstitution for parenteral use. It is suitable for hospital protocol, with physical and chemical stability maintained during storage and handling.
Quality Assured Packaging
This antibiotic is packed in double polyethylene bags within HDPE drums, preventing moisture and contamination. Each shipment is supported by a Certificate of Analysis, providing assurance on identity, purity, and compliance with safety standards.
FAQs of Isepamicin sulfate:
Q: How is Isepamicin sulfate used in clinical settings?
A: Isepamicin sulfate is administered parenterally, typically by injection, to treat bacterial infections. Its high purity and compliance with pharmacopeial standards make it suitable for use in hospitals and clinics.Q: What are the benefits of selecting Isepamicin sulfate for antibiotic therapy?
A: Isepamicin sulfate is highly pure, meets rigorous safety and quality standards, and is effective against a broad range of bacteria, enhancing patient outcomes and reducing infection risks.Q: When should Isepamicin sulfate be stored, and at what temperature?
A: Isepamicin sulfate should be stored at 2-8C in a dry, dark place, in tightly closed containers, to maintain its potency and prevent degradation.Q: Where can I find the Certificate of Analysis for my shipment?
A: A Certificate of Analysis is provided with every shipment of Isepamicin sulfate, ensuring full documentation of its pharmaceutical grade, purity, and compliance.Q: What is the process for reconstituting Isepamicin sulfate for injection?
A: The powder should be dissolved in sterile water for injection, following hospital protocols for preparing parenteral antibiotics. Its high solubility supports easy and consistent reconstitution.Q: How does Isepamicin sulfate meet pharmacopeial standards for safety?
A: It complies with USP/EP/JP requirements for microbial limits, endotoxins, heavy metals, loss on drying, and purity, ensuring safe use in medical applications.Q: Is Isepamicin sulfate suitable for export, and what are the packing details?
A: Yes, it is suitable for export. Packing is done in double polyethylene bags inside HDPE drums, typically of 1 kg, ensuring safe transportation and storage worldwide.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email

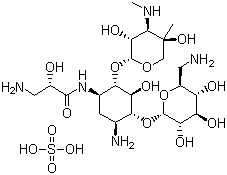

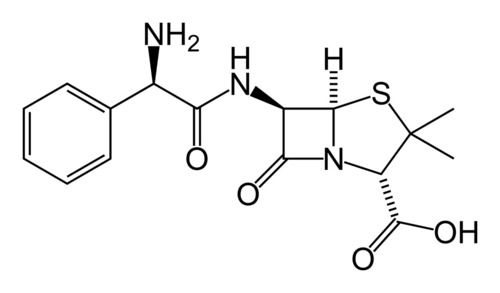
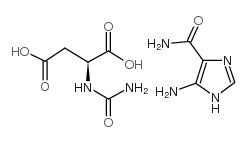
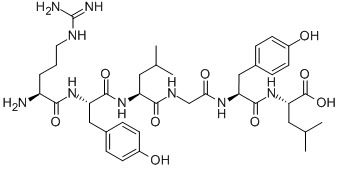

 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese